| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 7, July 2023, pages 237-243
Myocarditis Related to the Use of Mesalazine
Michaela Kyriakoua, Stefanos Sakellaropoulosb, Thrasos Constantinidesc, Grigorios Chatzantonisd, e, Panayiotis Avraamidesa, Andreas Mitsisa, f
aCardiology Department, Nicosia General Hospital, Nicosia, Cyprus
bDepartment of Internal Medicine, Cardiology Clinic, Kantonsspital Baden, Baden, Switzerland
cNicosia Heart Center, Nicosia, Cyprus
dDepartment of Cardiology I, University Hospital Munster, Munster, Germany
eDepartment of Cardiovascular MRI, Athens Medical Center, Athens, Greece
fCorresponding Author: Andreas Mitsis, Cardiology Department, Nicosia General Hospital, 2029 Nicosia, Cyprus
Manuscript submitted May 28, 2023, accepted July 1, 2023, published online July 31, 2023
Short title: Mesalazine-Induced Myocarditis
doi: https://doi.org/10.14740/jmc4104
| Abstract | ▴Top |
Myocarditis is a rare complication of therapy with mesalazine, a drug traditionally used in the treatment of inflammatory bowel disease. We report a case of a 32-year-old man with a recent diagnosis of ulcerative colitis, who presented to our hospital with chest pain and elevated troponin, 12 days following initiation of mesalazine. Diagnosis of myocarditis was confirmed with cardiac magnetic resonance imaging (CMR), which showed subepicardial gadolinium enhancement in the basal lateral/inferolateral segment of the heart. The patient’s clinical condition improved upon stopping mesalazine and the follow-up CMR demonstrated resolution of the previous findings. Mesalazine can cause myocarditis early after initiation and clinicians should be aware of this rare yet serious cardiotoxic effect, as the discontinuation of the medication is the mainstay of treatment and leads to significant recovery.
Keywords: Cardiac magnetic resonance; Mesalazine; Myocarditis; Inflammatory bowel disease; Ulcerative colitis
| Introduction | ▴Top |
Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), is a widely used and a well-established first-line treatment for inflammatory bowel disease (IBD). It belongs to the class of aminosalicylate drugs and exerts its therapeutic effects directly within the gastrointestinal system. The precise mechanism of action of the drug remains uncertain. However, the possible mechanism of action of mesalazine is the inhibition of cyclooxygenase (COX) enzyme, thereby impeding prostaglandin formation, that results in decreasing signaling via PPAR-γ pathway leading to decreased activity of nuclear factor κB [1]. By interrupting this cascade, mesalazine contributes to the reduction of colonic inflammation [1]. Upon oral administration, mesalazine is primarily metabolized in the gut, resulting in localized actions in that region. As a result, the incidence of systemic side effects is relatively low, with most adverse reactions being gastrointestinal in nature, such as abdominal pain, nausea, vomiting and diarrhea. Nevertheless, it is important to acknowledge that while rare, mesalazine can potentially give rise to certain complications beyond the gastrointestinal system. These less common adverse effects include pancreatitis, blood dyscrasias (abnormalities in blood cell counts or function), and cardiovascular complications [2, 3]. These manifestations may arise due to various mechanisms of action exhibited by mesalazine. Many potential sites of action include the inhibition of interleukin-2 and lipoxygenase, which are important inflammatory mediators [3].
Myocarditis, although rare, represents a significant and potentially hazardous complication of mesalazine therapy. Therefore, it is crucial to promptly recognize and differentiate it from extraintestinal manifestations of IBD. Early recognition and discontinuation of the medication are of utmost importance, as it can potentially save lives. In this context, we present a case study of a 32-year-old male patient who developed acute myocarditis shortly after initiating mesalazine treatment.
| Case Report | ▴Top |
Investigations
A 32-year-old male without prior cardiac history presented to the emergency department (ED) with an intense 8 out 10, central chest pain. The pain had been radiating to the left shoulder and the back over the previous 24 h. Concurrently, the patient experienced mild dyspnea and generalized weakness. There was no associated cough, fever, or other flu-like symptoms. Past medical history included a recent diagnosis of ulcerative colitis (UC), about 12 days ago, with the initiation of mesalazine 1.5 g b.i.d. On examination, the patient was afebrile and normotensive with normal oxygen saturation and normal heart rate. The cardiovascular and respiratory examination was unremarkable. Chest X-ray demonstrated clear lung fields with normal cardiothoracic ratio. Resting 12-lead electrocardiogram demonstrated sinus rhythm with concave ST-segment elevation in I, II, V4-6 (Fig. 1). Elevated cardiac biomarkers suggestive of myocardial damage were observed, with a troponin-I level of 6.53 ng/mL (normal range < 0.5 ng/mL) and with a CK-MB of 65 IU/L (normal range < 25 IU/L). In addition, further laboratory analysis revealed an elevated C-reactive protein (CRP) concentration of 105 mg/dL (normal range < 5 mg/dL) and others blood tests within normal parameters (including leucocytes and eosinophils concentrations). The transthoracic echocardiogram (TTE) demonstrated a normal-size left ventricle (LV) with normal systolic function with an ejection fraction (EF) of 55-60%. The global longitudinal strain was normal at 20.6%. Furthermore, the TTE did not show any evidence of pericardial effusion and no significant valvular disease was detected.
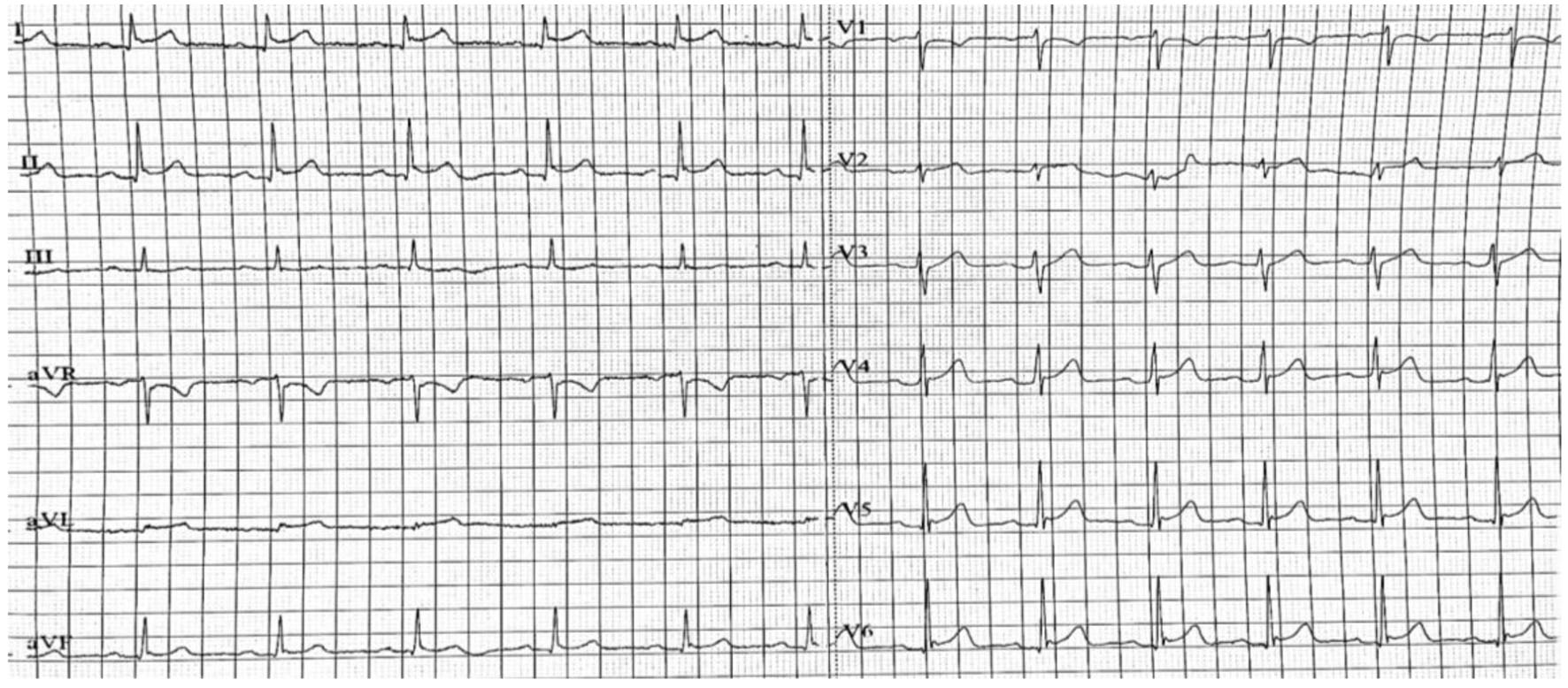 Click for large image | Figure 1. Resting 12-lead electrocardiogram demonstrating sinus rhythm with concave ST-segment elevation in I, II, V4-6. |
It is noteworthy to mention that during the first few days of admission, the patient experienced recurring episodes of chest pain. However, upon discontinuation of mesalazine, the symptoms subsided. The troponin-I levels demonstrated a characteristic decline, reaching 2.53 ng/mL, as measured 3 days after admission.
Diagnosis
Following the TTE, the patient underwent a coronary angiogram, which revealed unobstructed coronaries (Fig. 2a, b). Based on the above findings, the working diagnosis was acute myocarditis. Considering the patient’s clinical history, presentation and the absence of significant coronary artery disease, the differential diagnoses for the myocarditis included viral myocarditis, extraintestinal manifestation of UC, autoimmune processes, or drug-induced myocarditis. These possibilities highlight the importance of considering various etiologies in myocarditis cases and conducting a thorough evaluation.
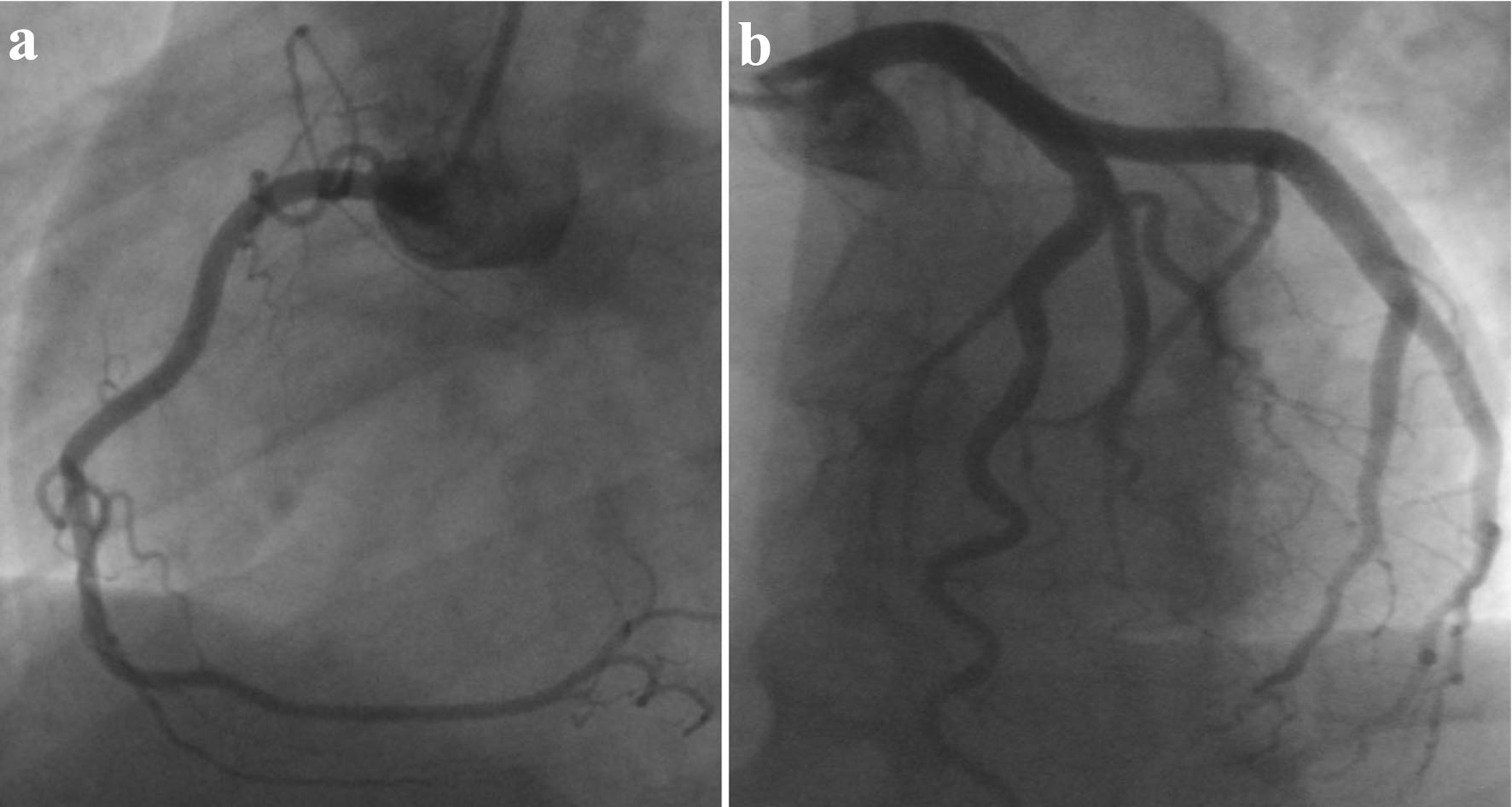 Click for large image | Figure 2. Coronary angiogram revealing normal coronaries, free of significant disease. (a) LAO view of RCA. (b) LAO cranial view of LCA. LAO: left anterior oblique; RCA: right coronary artery; LCA: left coronary artery. |
During the patient’s hospitalization, cardiac magnetic resonance imaging (CMR) was performed, which revealed high signal intensity on T2-weighted images in the basal lateral and apical septum wall segments (Fig. 3a, b), indicating the presence of myocardial edema. These findings are consistent with the inflammatory process associated with myocarditis. Additionally, subepicardial late gadolinium enhancement (LGE) was observed in the same areas (Fig. 3c, d). This finding indicates areas of scar tissue or fibrosis within the myocardium, which can occur because of inflammation and damage during myocarditis. Also, there were areas of patchy LGE distribution throughout the left ventricle. This pattern of enhancement is characteristic of myocarditis, as the inflammatory process often affects different areas of the myocardium in a non-uniform or patchy manner (Fig. 3). Importantly, despite the presence of these inflammatory and scar tissue findings, the CMR confirmed that the left ventricle was normal in size and demonstrated preserved ejection fraction.
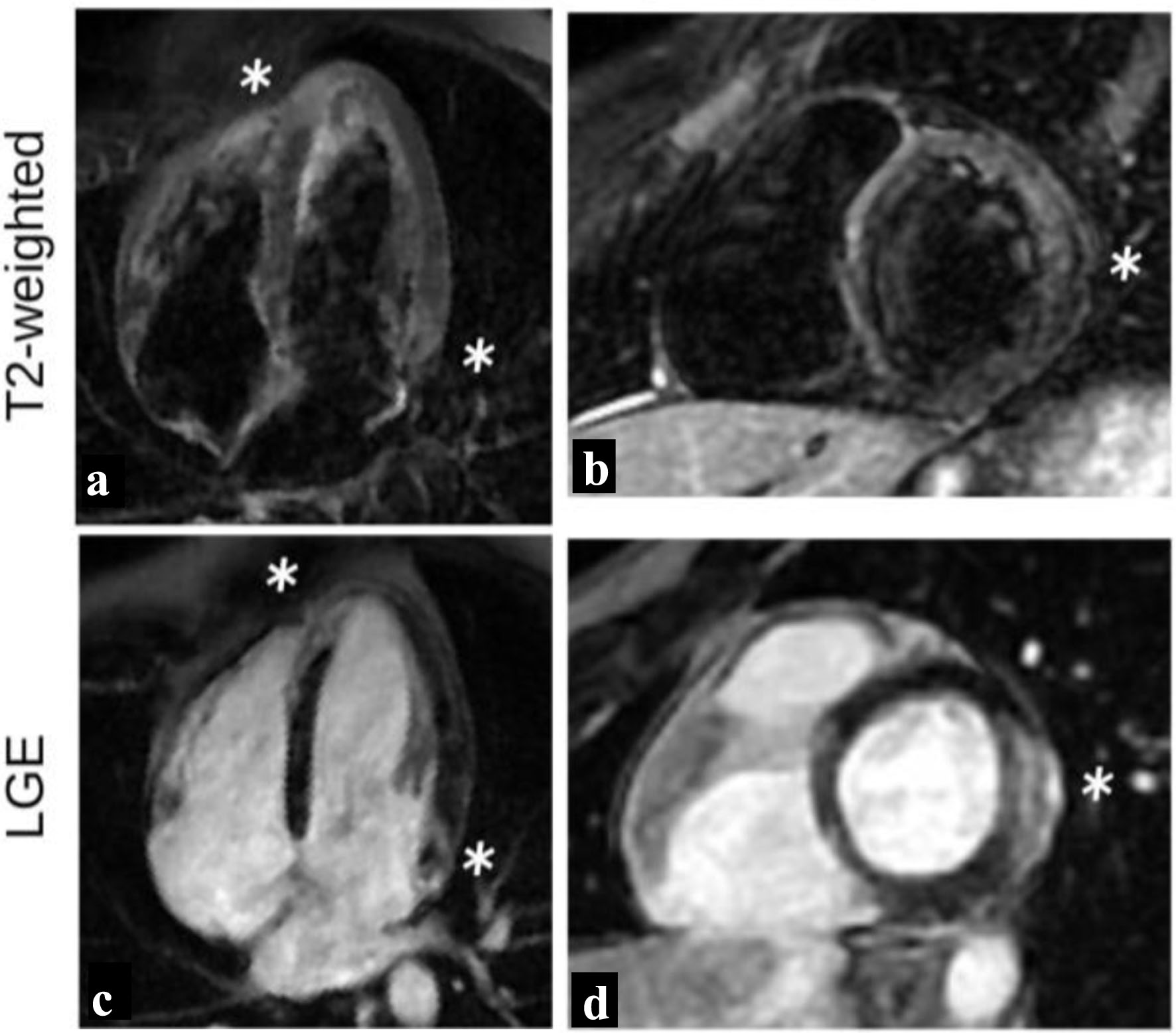 Click for large image | Figure 3. T2-weighted images and LGE distribution during initial study. High intensity signal (white stars) of the basal lateral and apical septum wall segments on T2-weighted images (a, b) indicating myocardial edema, with associated LGE of the same areas (c, d), findings indicative of acute myocarditis. LGE: late gadolinium enhancement. |
Based on a comprehensive evaluation incorporating the CMR findings, the temporal relationship between mesalazine initiation and the onset of cardiac symptoms, as well as the subsequent clinical improvement observed upon discontinuation of the medication, a conclusive diagnosis of mesalazine-induced myocarditis was made.
Treatment
In terms of symptomatic management, patient was treated with analgesia, while mesalazine was discontinued, as a working diagnosis of mesalazine-induced myocarditis was suggested.
Follow-up and outcomes
The patient was discharged a few days later, exhibiting complete resolution of symptoms and remaining entirely asymptomatic. As part of the discharge plan, a crucial recommendation was provided to the patient to abstain from lifelong usage of mesalazine. Subsequently, a follow-up CMR was conducted 4 months later, revealing a marked improvement and significant resolution of the previously observed findings (Fig. 4). This approach ensured appropriate patient care, monitoring, and management, resulting in the successful resolution of myocarditis-related symptoms and favorable cardiac recovery.
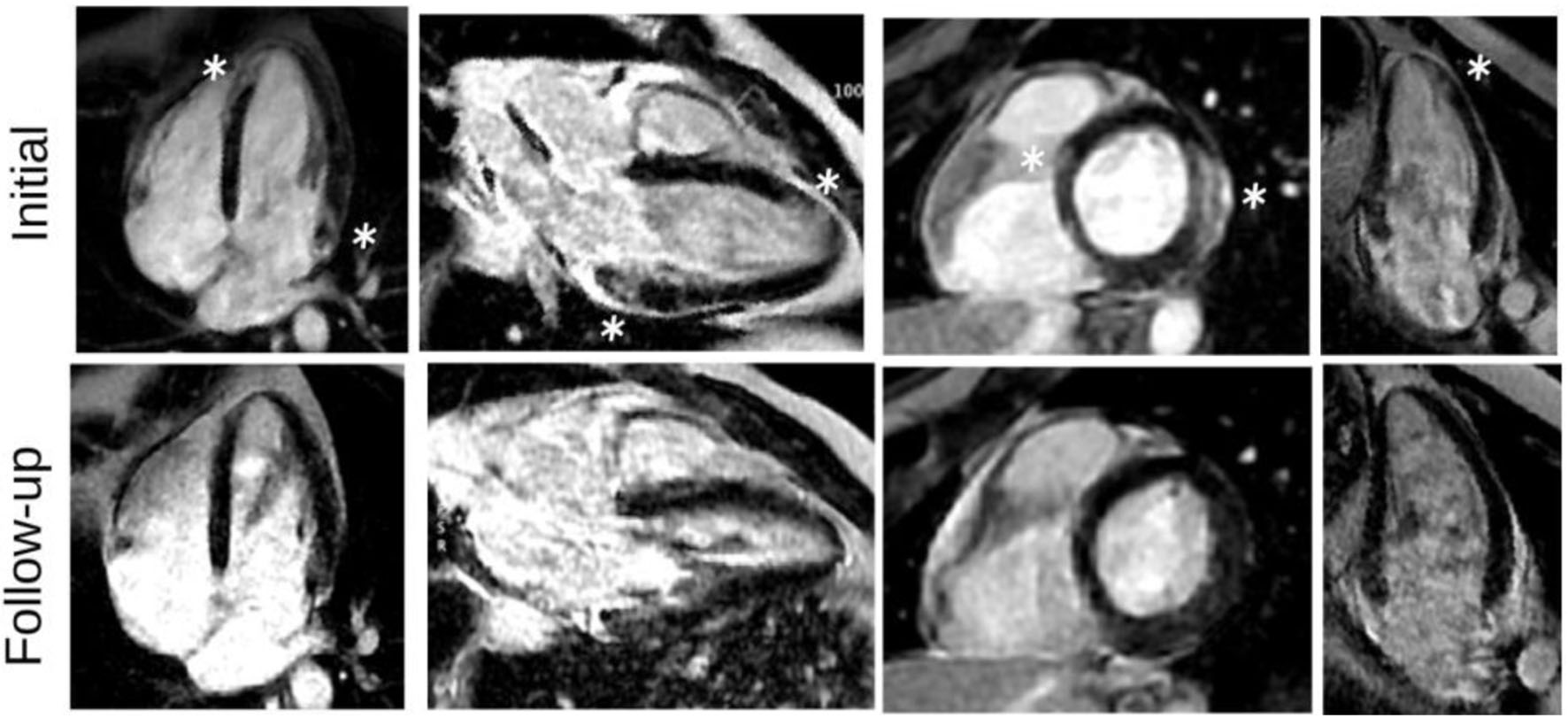 Click for large image | Figure 4. LGE images, during the initial presentation and in follow-up study. LGE images show subepicardial LGE of the basal lateral and apical septum wall segments (white stars), as well as patchy LGE distribution in a nonischemic pattern with significant improvement on follow-up. LGE: late gadolinium enhancement. |
| Discussion | ▴Top |
Myocarditis is an inflammatory disease of the myocardium and can manifest as an acute, subacute or chronic process [4]. It exhibits a diverse range of clinical presentations and outcomes, reflecting the polymorphic nature of the disease. Myocarditis primarily affects a young and healthy individual, with a higher prevalence among males [5]. It encompasses a broad spectrum of etiological factors, resulting from exposure to either discrete external antigens such as viruses, which are considered the most common cause, such as coxsackie, and also other microorganisms such as bacteria and parasites. However other external triggers, like toxins or drugs can cause myocardial inflammation. Furthermore, the disease can be activated by internal triggers, such as auto-immune conditions, as in systemic lupus erythematosus (SLE) [6]. This wide array of potential causes underscores the complex nature of myocarditis and its multifactorial origin. Clinical presentations include a wide range of symptoms, varying from mild fatigue, palpitations, and chest pain to more severe manifestations with the development of arrhythmias and syncopal episodes. In rare cases, more lethal complications may arise with the presence of fulminant myocarditis, which can be manifested as acute heart failure, cardiogenic shock, and even sudden cardiac death [7]. Approximately 20% of individuals diagnosed with myocarditis may later develop a chronic inflammatory dilated cardiomyopathy (DCM) [7]. In the majority of cases, myocardial inflammation gradually subsides and leads to complete resolution of the disease. However, in the specific case of DCM, persistent inflammation can initiate remodeling mechanisms that contribute to the degeneration of myocardial cells and the replacement of damaged myocardium with fibrotic tissue. As disease progresses, this fibrotic transformation can lead to myocardial dilatation, global remodeling of the left ventricle, and chronically reduced ejection fraction. These pathological changes ultimately characterize the clinical presentation of DCM. This phenomenon has been observed in animal models of myocarditis induced by viral infections and autoimmune conditions [8].
The diagnosis of myocarditis traditionally required a histologic diagnosis; however, endomyocardial biopsy remains largely underutilized in the clinical practice, as it possesses limitations with a diagnostic yield as low as 35% [9]. These limitations arise from the patchy distribution of inflammation observed in some cases, as well as the challenge of accessing the epicardial and mid-wall layers where inflammation often predominates [10]. Consequently, clinical suspicion together with laboratory and imaging criteria, such as CMR, has been used to secure the diagnosis of myocarditis.
Emerging evidence suggests a potential association between IBD and an increased risk of developing myocarditis compared to the general population [9, 11]. Although it is not entirely clear, it is thought to be related to the underlying inflammation that is characteristic of the disease, as inflammation can affect various parts of the body, including the heart, and may contribute to the development of the disease. Another, rare but plausible explanation is toxicity from mesalazine. The distinction between these causes can be difficult and demanding; however, it is crucial, as the early cessation of mesalazine can prevent any long-term dysfunction. The precise mechanisms, through which mesalazine may contribute to heart damage, are not fully understood. It is suggested that cell-mediated hypersensitivity reaction is the cause rather than a direct cardiotoxic effect. This is supported by the definitive resolution of disease following the discontinuation of the drug and by eosinophilic infiltration on endomyocardial biopsy specimens [11]. An additional supporting evidence of hypersensitivity reaction to mesalamine is the occurrence of rare cases presenting with hypersensitivity pneumonitis, angioedema, skin rashes, and hypereosinophilia [11]. Mesalazine has been shown to inhibit the activity of COX1 and enhance the metabolism of arachidonic acid into lipoxygenase products such as leukotrienes. The excessive production of lipoxygenase products can contribute to a pro-inflammatory signaling cascade, thereby promoting the development of allergic myocarditis [12]. This process involves the release of cytokines that stimulate eosinophils, leading to inflammation. Another potential mechanism is through humoral-mediated hypersensitivity response. In this scenario, antibodies produced against mesalazine can cross-react with heart tissues, leading to inflammation [13]. Furthermore, mesalazine has been found to induce the formation of reactive oxygen species, which disrupt mitochondrial membrane. This causes mitochondrial dysfunction and subsequent release of cytochrome c, eventually leading to cardiomyocyte apoptosis and eventually to cardiovascular dysfunction [12].
Sometimes it is challenging to confirm the diagnosis of myocarditis caused by mesalazine because there are no physical findings, symptoms, or laboratory tests that are pathognomonic for mesalazine-induced cardiotoxicity. The diagnosis is based on clinical features, with symptom onset typically occurring within 2 - 4 weeks of initiation of treatment and by the early resolution of symptoms with the discontinuation of the medication [14]. Simultaneously with the cessation of the medication, the clinicians should rule out other causes of myocarditis like cardiotoxicity of IBD, viral myocarditis and vasculitis.
Moreover, it is important to highlight that a recent retrospective study by Chen et al investigated the potential association between the dosage of mesalazine and the occurrence of myocardial injury. The study findings revealed no apparent link between the dosage of mesalazine and the occurrence of myocardial injury. Additionally, increasing the dose of mesalazine did not lead to a shorter onset time for cardiotoxicity [12]. These observations suggest that there is no apparent dose-dependent relationship between mesalazine and the development of myocardial injury or the timing of cardiotoxic manifestations.
Echocardiography is a valuable tool in the evaluation of acute myocarditis due to its ability to aid in distinguishing it from other conditions causing myocardial disease. However, there are no specific findings for acute myocarditis on TTE. The most common pattern is a dilated, spherical ventricle with reduced systolic function. Of note, a pericardial effusion usually signifies myopericarditis. In recent years, CMR has gained significant popularity for its effectiveness in detecting myocarditis. The visual manifestations of myocardial inflammation include the presence of edema, hyperemia, capillary leakage, necrosis, and fibrosis [15]. The intensity and severity of these alterations are based on the level of underlying myocardial inflammation and the timing of the CMR in relation to the natural course of the disease, as it progresses from an acute or subacute phase to a healed or chronic state. This temporal progression of myocardial inflammation, which often lasts days to weeks before eventual resolution, narrows the optimal window for diagnostic imaging sensitivity to only a few weeks from the initial onset of symptoms. So, performing CMR in the early stages of myocarditis is considered essential [16]. The assessment of CMR findings in myocardial inflammation is frequently done using the revised Lake Louise Criteria (LLC). According to the revised criteria, CMR provides strong evidence of acute myocardial inflammation for the definite diagnosis of myocarditis, in patients with a high clinical pretest probability. It demands at least one T1-based marker of nonischemic myocardial injury (abnormal T1 mapping, increased extracellular volume, or LGE) and at least one T2-based marker of myocardial edema (abnormal T2 mapping or regional abnormalities on T2-weighted imaging) [17]. Myocardial edema arises as a consequence of acute inflammation during the initial phases of myocarditis and is commonly observed in the septum or lateral wall in a non-vascular distribution, although any myocardial wall can be affected. In contrast to edema seen in acute infarction, which primarily involves the subendocardial layer, edema associated with myocarditis tends to spare this region and predominantly affects the subepicardial and mid-myocardial layers of the cardiac tissue. Additionally, the detection of LGE indicates the presence of tissue inflammation, necrosis and early fibrosis. The presence of fibrosis is widely acknowledged as a significant contributor to the development of ventricular arrhythmias, as it promotes the formation of re-entrant circuits and also has been associated with an increased risk of LV remodeling, heart failure and sudden cardiac death. Worsened outcomes have been associated with a larger extent of LGE in myocardial imaging, defined as either LGE involvement in more than two LV segments, LGE exceeding 10% of LV mass, or LGE exceeding 17 g [18]. In addition, the presence of LGE in the anteroseptal region has also been linked to unfavorable clinical outcomes [18]. These findings highlight the significance of LGE as an important marker in myocardial imaging for risk stratification and prognostic evaluation in patients with myocarditis.
Conclusion
Mesalazine is established as the primary treatment for patients with mild to moderate UC and remains the cornerstone of maintenance therapy. In the context of IBD, cardiac involvement can manifest as an extraintestinal manifestation or may be associated with mesalazine treatment itself. Mesalazine-induced myocarditis typically presents within a few days to weeks following initiation of the medication.
When a patient presents with cardiac symptoms and mesalazine-induced myocarditis is suspected, CMR is recommended as a diagnostic modality. CMR aids in establishing the diagnosis by visualizing inflammation in both the myocardium and pericardium.
Timely recognition and diagnosis of mesalazine-induced myocarditis are of utmost importance due to its potential life-threatening nature. Upon confirmation of the diagnosis, prompt discontinuation of mesalazine is advised. Following cessation of the medication, symptoms generally resolve, and cardiac function can improve.
Learning points
Myocarditis is a rare, underdiagnosed complication of mesalazine, which occurs early after initiation. Clinicians should be wise to this rare yet life-threating cause of myocarditis and establish the diagnosis on time, as the early cessation of mesalazine would lead to resolution of the symptoms and can improve cardiac function.
CMR is considered the gold standard non-invasive diagnostic modality for confirming the diagnosis of mesalazine-induced myocarditis. Its utilization not only aids in establishing an accurate diagnosis but also plays a pivotal role in guiding clinical decision-making and subsequent follow-up.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
AM participated in patient care, writing of the case report, revisions, and submission process. MK and PA participated in patient care, writing of the case report and revisions. TC and GC participated in imaging. SS participated in writing of the case reports and revisions.
Data Availability
All data in our report were obtained from the patient’s hospitalization. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Perez-Colon E, Dadlani GH, Wilmot I, Miller M. Mesalamine-induced myocarditis and coronary vasculitis in a pediatric ulcerative colitis patient: a case report. Case Rep Pediatr. 2011;2011:524364.
doi pubmed pmc - Sehgal P, Colombel JF, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther. 2018;47(12):1597-1609.
doi pubmed - Iacucci M, de Silva S, Ghosh S. Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol. 2010;24(2):127-133.
doi pubmed pmc - Lampejo T, Durkin SM, Bhatt N, Guttmann O. Acute myocarditis: aetiology, diagnosis and management. Clin Med (Lond). 2021;21(5):e505-e510.
doi pubmed pmc - Golpour A, Patriki D, Hanson PJ, McManus B, Heidecker B. Epidemiological Impact of Myocarditis. J Clin Med. 2021;10(4):603.
doi pubmed pmc - Cooper LT, Jr. Myocarditis. N Engl J Med. 2009;360(15):1526-1538.
doi pubmed pmc - Tschope C, Cooper LT, Torre-Amione G, Van Linthout S. Management of myocarditis-related cardiomyopathy in adults. Circ Res. 2019;124(11):1568-1583.
doi pubmed - Fairweather D, Beetler DJ, Musigk N, Heidecker B, Lyle MA, Cooper LT, Jr., Bruno KA. Sex and gender differences in myocarditis and dilated cardiomyopathy: An update. Front Cardiovasc Med. 2023;10:1129348.
doi pubmed pmc - Ammirati E, Buono A, Moroni F, Gigli L, Power JR, Ciabatti M, Garascia A, et al. State-of-the-art of endomyocardial biopsy on acute myocarditis and chronic inflammatory cardiomyopathy. Curr Cardiol Rep. 2022;24(5):597-609.
doi pubmed pmc - Vidusa L, Kalejs O, Maca-Kaleja A, Strumfa I. Role of endomyocardial biopsy in diagnostics of myocarditis. Diagnostics (Basel). 2022;12(9):2104.
doi pubmed pmc - Merceron O, Bailly C, Khalil A, Pontnau F, Hammoudi N, Dorent R, Michel PL. Mesalamine-induced myocarditis. Cardiol Res Pract. 2010;2010:930190.
doi pubmed pmc - Chen J, Duan T, Fang W, Liu S, Wang C. Analysis of clinical characteristics of mesalazine-induced cardiotoxicity. Front Pharmacol. 2022;13:970597.
doi pubmed pmc - Okoro KU, Roby MD, Bankole AA. Myocarditis secondary to mesalamine-induced cardiotoxicity in a patient with ulcerative colitis. Case Rep Med. 2018;2018:9813893.
doi pubmed pmc - Haq MI, Ahmed S, Pasha W, Zaidi SAA. Mesalazine-induced cardiotoxicity. J Rare Disord Diagn Ther. 2019;4:24.
doi - Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158-3176.
doi pubmed - Polte CL, Bobbio E, Bollano E, Bergh N, Polte C, Himmelman J, Lagerstrand KM, et al. Cardiovascular magnetic resonance in myocarditis. Diagnostics (Basel). 2022;12(2):399.
doi pubmed pmc - Gutberlet M, Lucke C. Original versus 2018 Lake Louise criteria for acute myocarditis diagnosis: old versus new. Radiol Cardiothorac Imaging. 2019;1(3):e190150.
doi pubmed pmc - Georgiopoulos G, Figliozzi S, Sanguineti F, Aquaro GD, di Bella G, Stamatelopoulos K, Chiribiri A, et al. Prognostic impact of late gadolinium enhancement by cardiovascular magnetic resonance in myocarditis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2021;14(1):e011492.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


