| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 1, January 2023, pages 36-43
Uncommon Presentation of Undiagnosed B-Cell Lymphoproliferative Disorder as Nodular Pulmonary Amyloidosis
Harsh Patela, o, Aaiyat Sheikhb, Gnana Deepthi Medarametlac, Sri Abirami Selvamd, Syed Nazeer Mahmoode, Gurleen Johalf, Janani Arunachalamg, Haripriya Radhakrishnanh, Viray Shahi, Aditya Lal Vallathj, Digantkumar Patelk, Saketh Palasamudram Shekarl, Urvish Patelm, Nisarg Changawalan
aDepartment of Family Medicine, Central Jersey Urgent Care, Green Brook, NJ 08812, USA
bEra’s Lucknow Medical College, Lucknow, Uttar Pradesh 226003, India
cNRI Medical College and General Hospital, Guntur, Andhra Pradesh 522503, India
dDepartment of Internal Medicine, St Mary Medical Center, Langhorne, PA 19047, USA
eDepartment of Medicine, Section of Pulmonary/Critical Care, MedStar Washington Hospital Center, Washington, DC 20770, USA
fDepartment of Medicine, Hackensack Meridian Palisades Medical Center, North Bergen, NJ 07047, USA
gDepartment of Biomedical Engineering, University of Houston, Houston, TX 77021, USA
hShimoga Institute of Medical Sciences, Shimoga, Karnataka 577201, India
iDepartment of Hospital Medicine, Medstar Good Samaritan Hospital, Baltimore, MD 21239, USA
jDepartment of Emergency Medicine, Peerless Hospital and BK Roy Research Center, Kolkata, West Bengal 700094, India
kSpringfield Memorial Hospital, Springfield, IL 62781, USA
lInterventional Pulmonology, Department of Pulmonary and Critical care Medicine, Pulmonary and Sleep Associates of Huntsville, Huntsville Hospital, Huntsville, AL 35801, USA
mDepartment of Public Health and Neurology, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA
nThe Lung Center of Nevada, Las Vegas, NV 89138, USA
oCorresponding Author: Harsh Patel, Department of Family Medicine, Central Jersey Urgent Care, Green Brook, NJ 08812, USA
Manuscript submitted November 21, 2022, accepted January 11, 2023, published online January 18, 2023
Short title: Uncommon B-Cell Lymphoproliferative Disorder
doi: https://doi.org/10.14740/jmc4026
| Abstract | ▴Top |
B-cell lymphoproliferative disorders are characterized by the accumulation of mature B lymphocytes in the bone marrow, lymphoid tissues, and/or peripheral blood. They can cause amyloid deposits in the lungs. In rare cases, lung nodules can be the first sign of this disorder. We present the case of an 89-year-old woman with stable shortness of breath and lung nodules on imaging. A positron emission tomography-computed tomography (PET-CT) scan showed the most intense hypermetabolic nodule in the patient’s lung, which was 1.5 × 1.4 cm. A biopsy of this nodule showed amyloid material with trapped plasma cell infiltrate on microscopy. Congo red stain under polarizing microscopy showed apple-green birefringence, which is diagnostic for amyloidosis. Immunohistochemistry showed a mixture of kappa-positive and lambda-positive cells. B-cell gene rearrangement-clonal gene rearrangements were detected in the immunoglobulin heavy chain (IgH) gene and the kappa light chain (IGK). These findings suggest a B-cell lymphoproliferative disorder, such as a plasmacytoma or a marginal cell lymphoma with plasma cell differentiation. The patient was diagnosed with a B-cell lymphoproliferative disorder and pulmonary amyloidosis. Isolated amyloidosis in the lungs usually has a good prognosis, but it can be a sign of autoimmune diseases or B-cell lymphoproliferative disorders, as in this case. Early diagnosis of B-cell lymphoproliferative disorder can lead to successful treatment and prevents complications.
Keywords: Nodular pulmonary amyloidosis; Pulmonary amyloidosis; B-cell lymphoproliferative disorder; Pulmonology; Oncology; Evidence based medicine
| Introduction | ▴Top |
Amyloidosis is a rare disorder caused by amyloid material building up in organs or tissues and causing organ dysfunction or death. It can affect multiple organs (systemic) or just one organ (localized). Localized amyloidosis usually affects the skin, bladder, or lungs. There are three types of amyloidosis in the lungs: diffuse alveolo-septal, nodular, and tracheobronchial. Most cases of amyloidosis in the lungs do not cause symptoms and are found by accident. Localized amyloidosis has a good prognosis, with a 90.6% survival rate of 5 years [1]. The incidence of systemic amyloidosis is about 14 cases per 1,000,000 people per year. However, localized amyloidosis is rare, and its estimated incidence is unknown [2].
Computed tomography (CT) scans can show different types of amyloidosis in the lungs: tracheobronchial, diffuse alveolar septal, and nodular parenchymal [3]. Positron emission tomography (PET) scans usually show positive fluorodeoxyglucose (FDG) uptake in nodular pulmonary amyloidosis [4]. Compared to transbronchial needle aspiration, radiology-guided fine-needle aspiration biopsy of a peripheral pulmonary nodule has a higher yield for pathologic diagnosis [5]. Localized amyloidosis can be managed without treatment, while systemic amyloidosis can be treated with chemotherapy and anti-inflammatory drugs [6].
B-cell lymphoproliferative disorders are characterized by the accumulation of mature B lymphocytes in the bone marrow, lymphoid tissues, and/or peripheral blood. B-cell lymphoproliferative disorders are diagnosed with flow cytometric immunophenotyping that shows clonal light-chain (kappa or lambda) restricted population showing B-cell markers in the blood or bone marrow [7]. This case illustrates a B-cell lymphoproliferative disorder associated with nodular pulmonary amyloidosis, presenting as pulmonary nodules. The patient had no other symptoms or signs of this disorder. A lung nodule biopsy showed both amyloidosis and a B-cell lymphoproliferative disorder.
| Case Report | ▴Top |
Investigations
An 89-year-old woman with a past medical history of pulmonary arterial hypertension, atrial fibrillation, and ischemic stroke presented to the office to establish care for chronic stable exertional dyspnea for many years. The patient had no associated cough, wheezing, chest pain, or palpitations. The patient did not have a history of smoking cigarettes, alcohol, or illicit drugs. Her family history was significant for stroke and uterine cancer in her mother, multiple myeloma in two of her brothers, and renal cell cancer in her sister. Further investigation into history led to the discovery of a CT chest performed 4 years prior that showed multiple bilateral calcified and noncalcified lung nodules. The patient did not have any other further workup at that time.
Diagnosis
A plain chest X-ray performed at the presentation showed extensive pulmonary nodulo-reticular shadowing at the peripheries signifying extensive fibrotic changes with scattered nodules (Fig. 1).
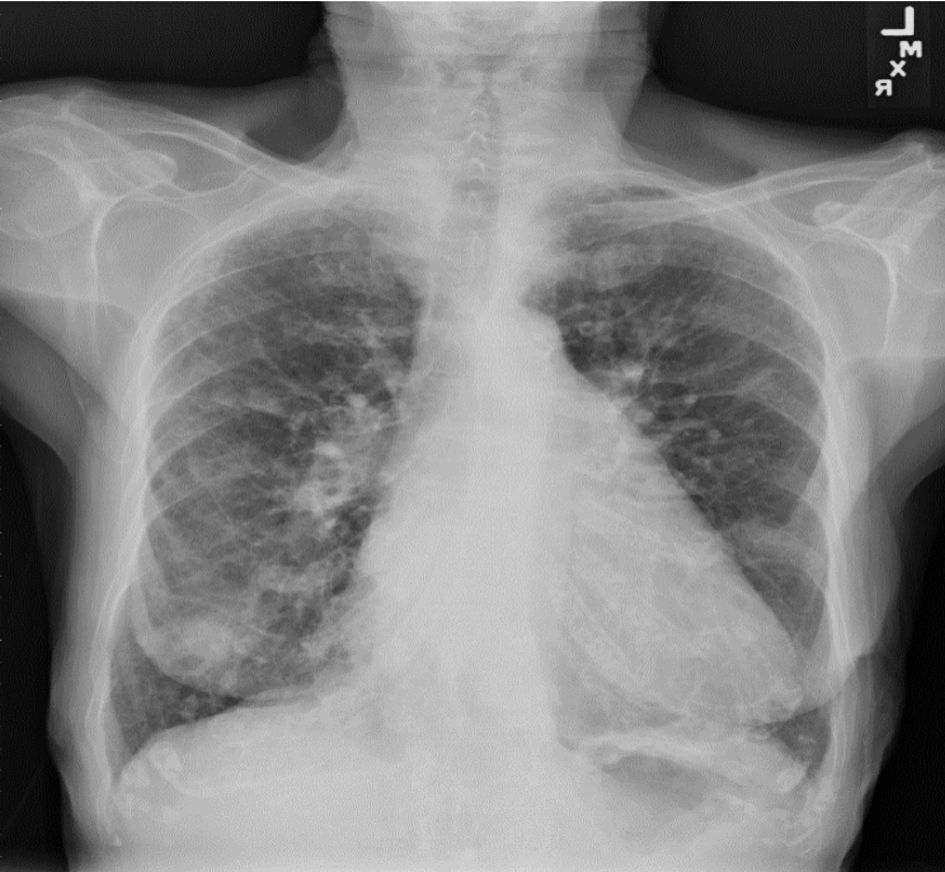 Click for large image | Figure 1. Chest X-ray showed extensive pulmonary nodulo- reticular shadowing at the peripheries signifying extensive fibrotic changes with scattered nodules. |
Subsequently, a CT chest performed in March 2022 (Fig. 2) showed several nodules bilaterally, demonstrating an interval increase in size compared to the prior study. A calcified nodule in the apical segment of the left lower lobe demonstrated increased soft tissue component measuring at 1.4 cm, compared to 1.2 cm in the prior study. Along the posterior mediastinal pleural surface of the left lung, above the level of the aortic knob, a 2-cm calcified nodule was spotted, unchanged since the prior study. An interval increase in the size of the nodular density in the right apex, as well as the development of several new nodules was also noted.
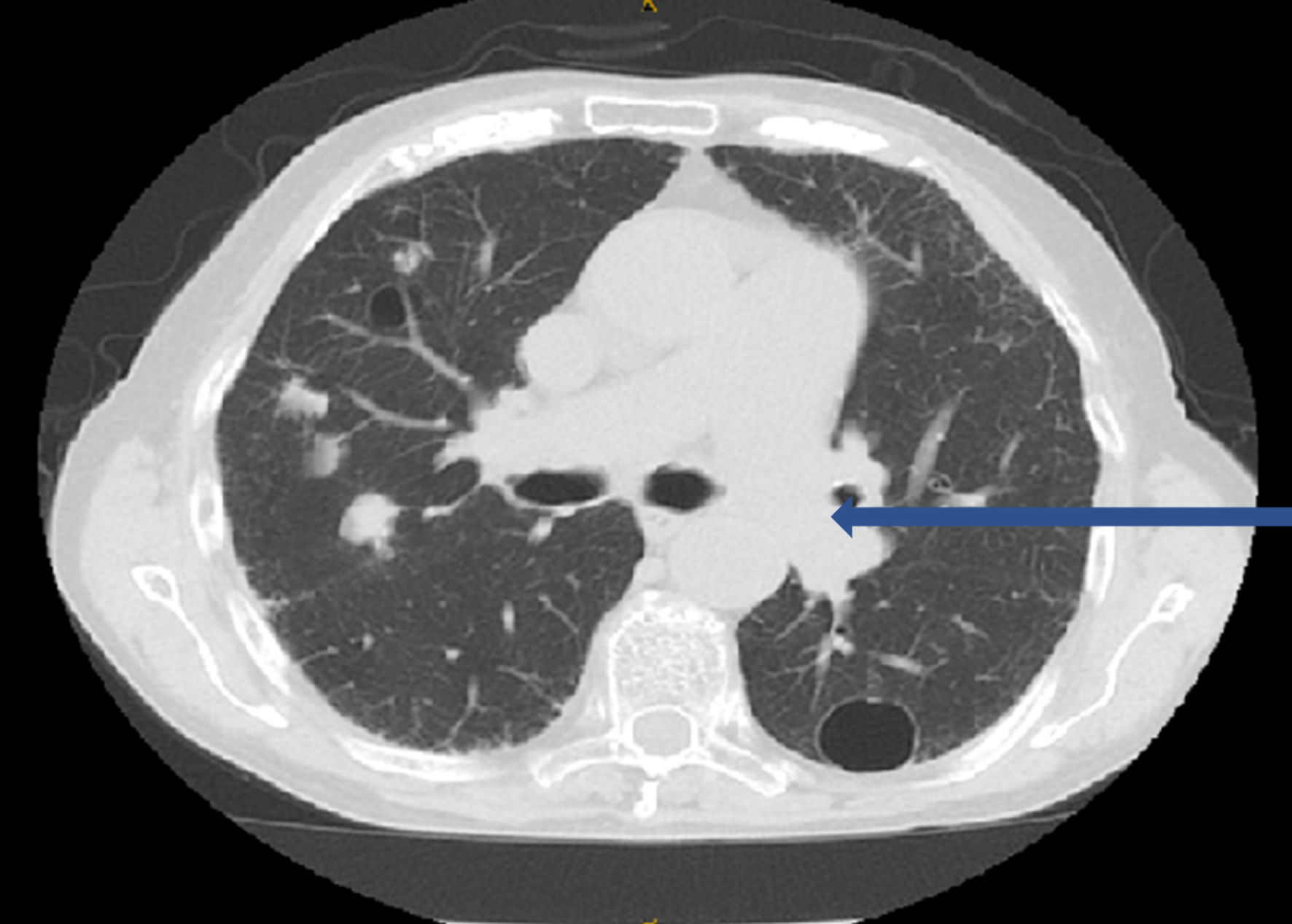 Click for large image | Figure 2. CT chest showed several nodules bilaterally: a calcified nodule (1.4 cm) in the apical segment of the left lower lobe and a 2-cm calcified nodule along the posterior mediastinal pleural surface of the left lung (blue arrow), above the level of the aortic knob. CT: computed tomography. |
Coccidioides antibody level, complement fixation (CF) was within normal limit (WNL) at < 1:2. A PET-CT scan was performed to direct appropriate lung nodule biopsy. It showed multiple bilateral mildly hypermetabolic pulmonary nodules. The most intense was in the posterior right upper lobe measuring 1.5 × 1.4 cm. Another representative area is a nodule in the lateral right middle lobe of the lungs measuring 2.4 × 2.0 cm with a max standardized uptake value (SUV) of 1.9.
A CT-guided needle biopsy of FDG avid right upper lobe (RUL) lung nodule was performed. Pathology showed homogenous acellular amorphous material with an entrapped plasma cell infiltrate on microscopy (Fig. 3a-c). Congo red stain using polarizing microscopy confirmed the presence of apple-green birefringence (Fig. 4a, b). Hematoxylin and eosin (H&E) and Congo red findings were diagnostic of amyloidosis. Immunohistochemistry showed a mixture of kappa-positive (Fig. 5) and lambda-positive (Fig. 6) cells but the predominance of kappa-positive cells. These findings suggest an atypical plasma cell population, and a tissue block was sent for B-cell rearrangement studies to diagnose plasma cell neoplasm. B-cell gene rearrangement-clonal gene rearrangements were detected in the immunoglobulin heavy chain gene (IgH) and the kappa light chain gene (IGK).
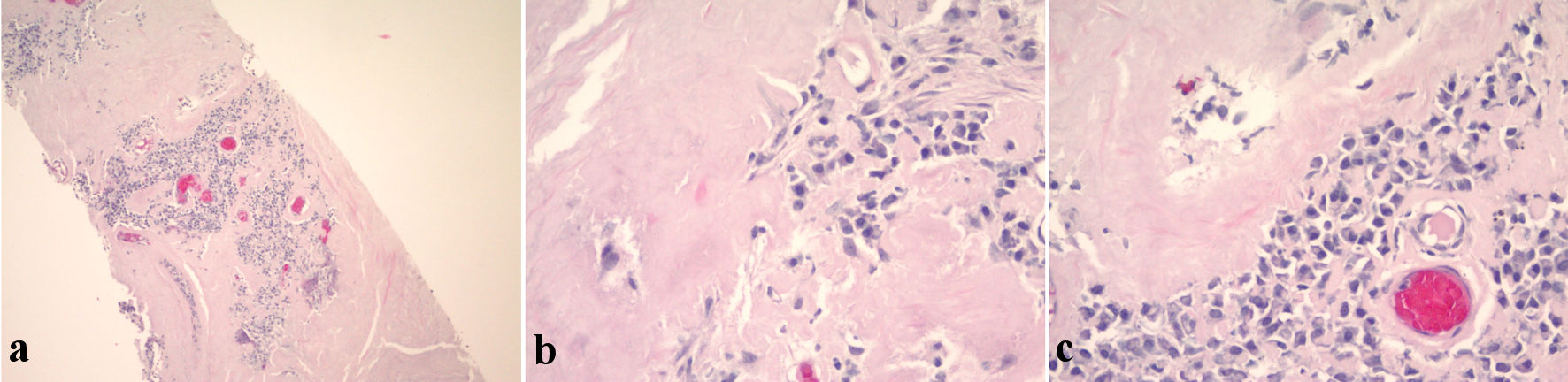 Click for large image | Figure 3. (a) Low magnification H&E stain. (b) High magnification H&E stain showing homogenous acellular amorphous material (amyloid) with plasma cells. (c) High magnification H&E stain showing homogenous acellular amorphous material (amyloid) with plasma cells. H&E: hematoxylin and eosin. |
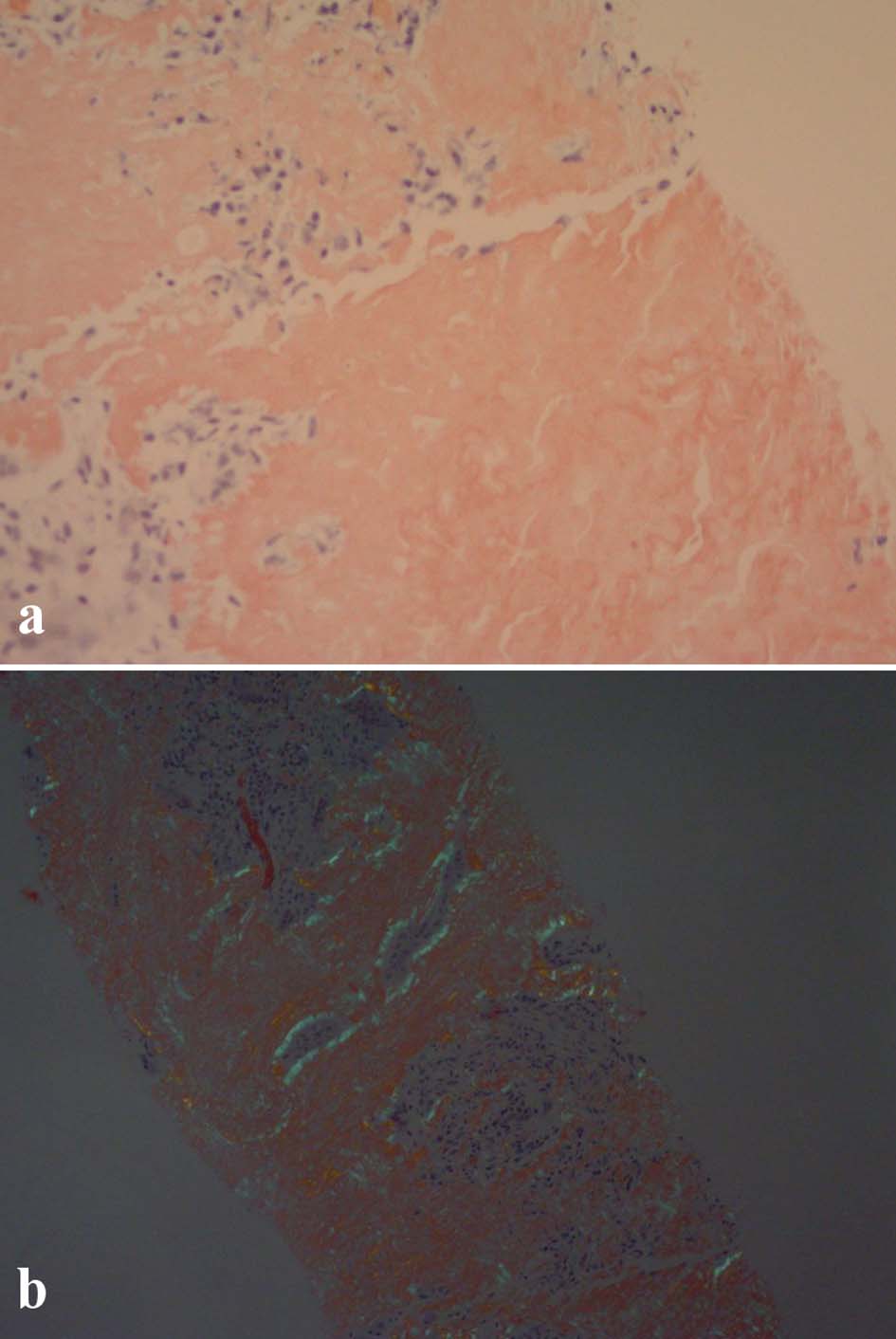 Click for large image | Figure 4. (a) Unpolarized Congo red stain. (b) Polarized Congo red stain showing apple-green birefringence. |
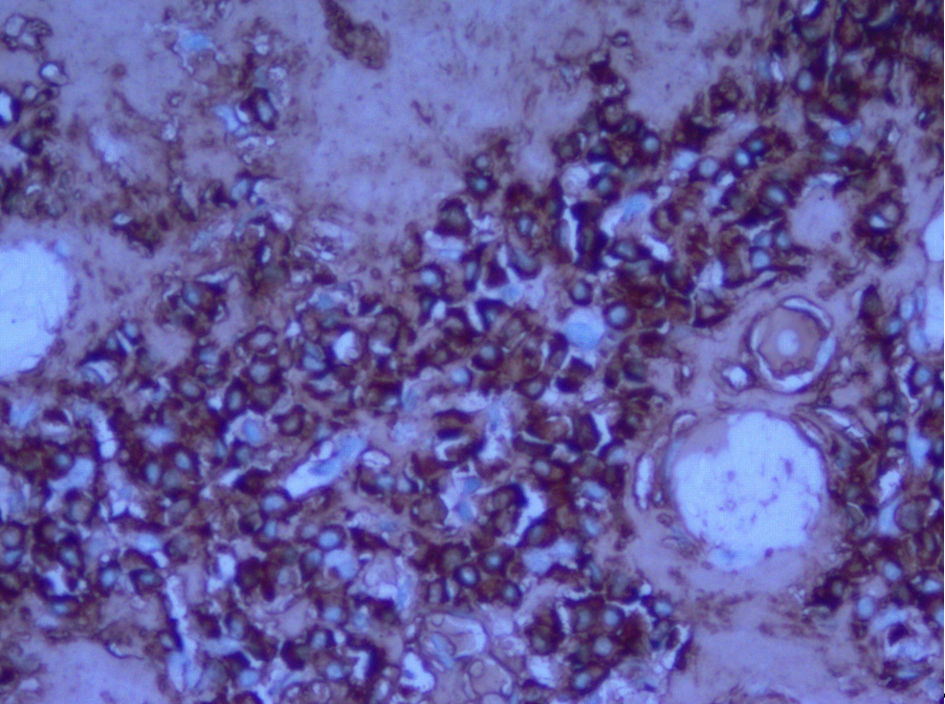 Click for large image | Figure 5. Immunohistochemistry showing kappa light chains. |
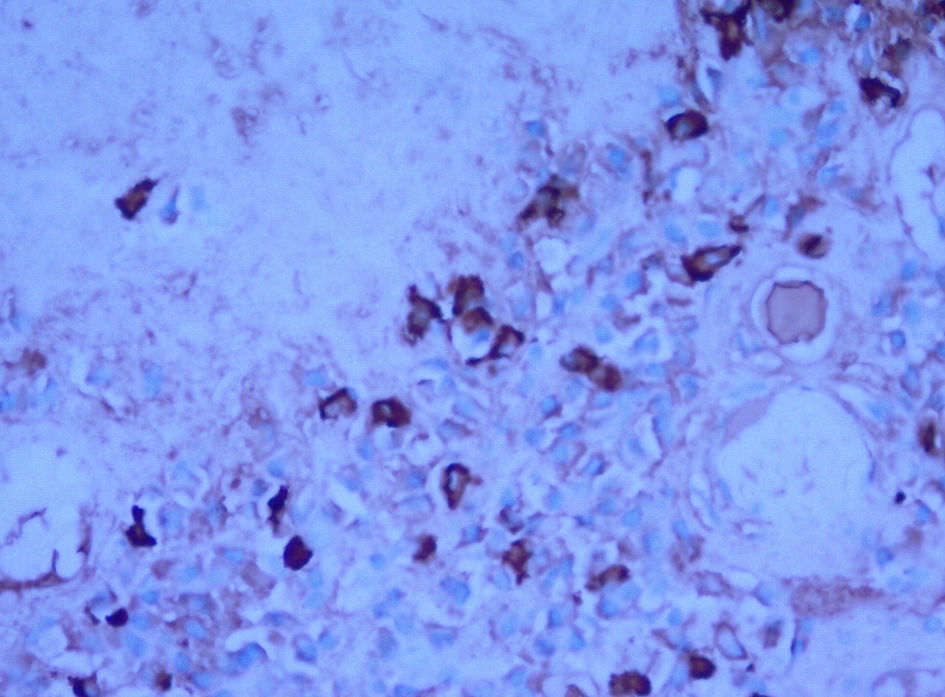 Click for large image | Figure 6. Immunohistochemistry showing lambda light chains. |
Treatment
These results were highly suggestive of a B-cell lymphoproliferative disorder, such as a plasmacytoma or a marginal cell lymphoma with plasma cell differentiation. The patient was not prescribed any treatments.
Follow-up and outcomes
The patient was subsequently referred to oncology for further management.
| Discussion | ▴Top |
Amyloidosis is usually caused by a protein called amyloid precursor protein (APP) being produced in excess or not being broken down properly. It can be inherited or acquired. Inherited amyloidosis is rare and is caused by a gene mutation. Acquired amyloidosis is more common and can be caused by chronic inflammation, multiple myeloma, and certain other blood disorders. Amyloidosis can also be caused by other proteins, such as transthyretin, in rare cases. Transthyretin amyloidosis is usually inherited and can affect the heart. Systemic amyloidosis is caused by changes in proteins and deposits as fibrils in tissues [8, 9]. It is not common for amyloidosis to only affect one area of the body, such as the lungs. In 1877, Lesser was the first who described finding an insoluble fibrillar protein deposit in the lungs during an autopsy [10]. There are three types of amyloidosis in the lungs: tracheobronchial, alveolar septal, and nodular [11].
A study by Mayo Clinic reported a case series of 55 patients with amyloidosis in the lungs from 1980 to 1993. In a study, out of 11 patients with amyloidosis in only their lungs, seven had nodular amyloidosis, and four had tracheobronchial amyloidosis [12]. This means that nodular pulmonary amyloidosis is a slightly more common pulmonary amyloidosis. CT scans of the chest can show multiple or single nodules in the lower part of the lungs and near the surface of the lungs, i.e., a subpleural region in nodular amyloidosis [13]. The average age of diagnosis is 67, and it is more common in men [14]. Nodular pulmonary amyloidosis is a disease that is often asymptomatic and detected incidentally; however, some people with this disease may experience symptoms such as wheezing, coughing, musical breathing, and recurrent pneumonia. It is important to consider nodular pulmonary amyloidosis in patients with multiple lung nodules [15]. Our patient, in this case, had chronic, stable shortness of breath and multiple pulmonary nodules on earlier imaging tests.
Nodular pulmonary amyloidosis is often misdiagnosed as neoplasm. Other possible causes of multiple lung nodules include infections, tumors, pneumoconiosis, sarcoidosis, rheumatoid arthritis, amyloidosis, and pulmonary alveolar microlithiasis. Tumors such as metastasis or primary lung cancer may cause multiple lung nodules. Infections that may lead to multiple pulmonary nodules include fungal infections like histoplasmosis, coccidioidomycosis, and aspergillosis [16, 17]. Other conditions like tumors and infections usually get worse quickly, but that was not the case for our patient.
A PET-CT with fluorine-18 fluorodeoxyglucose (18F-FDG) can show areas of increased cell activity. It is accurate for finding the cause of lung nodules and guiding a biopsy [18]. In our case, the scan showed the most intense hypermetabolic nodule in the patient’s lung, which was 1.5 × 1.4 cm, guided to a CT-guided needle biopsy of this lung nodule. The gold standard test to detect amyloid material is immunohistochemistry. Amyloid material looks like a homogeneous, acellular, and eosinophilic protein under a microscope. A special Congo red stain, viewed under polarized light, shows a green color [13]. Our patient’s pathology showed homogenous acellular amorphous material with an entrapped plasma cell infiltrate on microscopy. Congo red stain using polarizing microscopy confirmed the apple-green birefringence, which is diagnostic of amyloidosis.
Symptoms can guide treatment for amyloid deposits in the lungs. Patients who do not have symptoms usually do not need treatment. Various treatment options include surgical resection, resection by bronchoscopy, Nd:YAG laser therapy, and carbon dioxide laser ablation. Parenchymal amyloid nodules in the lungs usually grow slowly and do not cause symptoms, so they usually do not need treatment. Some drugs are under trial that stabilize amyloid precursor proteins and accelerate the tissue clearance of amyloid fibrils. Our patient was not given any treatment and was referred to a cancer specialist for more testing [11].
A study by Mahmood et al (2015) found that out of 606 patients with localized amyloidosis, including 35 patients with tracheobronchial amyloidosis and 47 patients with nodular pulmonary amyloidosis, only seven of them developed systemic amyloid after an average of 74 months of follow-up. The survival rate for 5 years was 90% and for 10 years was 80%. Localized amyloidosis usually has a good prognosis and does not often turn into systemic amyloidosis [19].
Lymphoproliferative disorders are conditions where certain types of blood cells (e.g., B-cells or T-cells) grow too much. Depending on the specific disorder, they can have different symptoms, pathologic findings, test results, and images. They can be either not cancerous or cancerous, including blood cancer-like lymphomas [20]. Flow cytometry can diagnose these disorders by looking at cells from a fine needle aspiration [21].
When biopsy specimen of the lung nodule was examined under a microscope, it showed certain types of blood cells. We did more testing on the sample and found that it had a mixture of kappa-positive and lambda-positive cells, with more kappa-positive cells. This suggested that the patient had an atypical type of plasma cell. Then, a tissue block was sent for B-cell rearrangement studies to diagnose plasma cell neoplasm. Test results showed changes in specific genes in the patient’s blood cells, which suggested they had B-cell lymphoproliferative disorder. This explained the multiple lung nodules the patient had. They were diagnosed with this disorder and referred to a cancer specialist for further testing and treatment.
Here we are giving a comprehensive review of similar case reports published by other authors (Table 1) [22-30].
 Click to view | Table 1. A Comprehensive Review of Similar Case Reports Published by Other Authors |
In conclusion, a B-cell lymphoproliferative certain kind of blood cell disorder can cause amyloid deposits in the lungs. In rare cases, it can first show as lung nodules. In our case, the patient had lung nodules and trouble breathing for a long time. She was tested following standard procedures, and it was found that her lung nodules were caused by B-cell lymphoproliferative disorder.
Learning points
B-cell lymphoproliferative disorder can present as pulmonary amyloidosis. In rare undiagnosed cases, the first manifestation can be pulmonary nodules. Isolated pulmonary amyloidosis has a good prognosis but should trigger an evaluation for autoimmune diseases and B cell lymphoproliferative disorders, as in this case, with early referral and treatment of the underlying condition. Early diagnosis of B-cell lymphoproliferative disorder can lead to successful treatment and prevents complications.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no financial disclosure.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was obtained by the patient for this case report.
Author Contributions
Acquisition of data: Nisarg Changawala. Conception or design of the work: Harsh Patel, Janani Arunachalam, Haripriya Radhakrishnan. Drafting of the manuscript: Harsh Patel, Aaiyat Sheikh, Gnana Deepthi Medarametla, Sri Abirami Selvam, Gurleen Johal, Aditya Lal Vallath. Revisions: Harsh Patel, Nisarg Changawala. Supervision: Syed Nazeer Mahmood, Viray Shah, Digantkumar Patel, Saketh Palasamudram Shekar, Urvish Patel, Nisarg Changawala.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Mnacakanova E, Henzlova L, Flodrova P, Pika T. Localised forms of pulmonary amyloidosis. Vnitr Lek. 2021;67(6):357-364.
doi pubmed - Edriss, et al. Isolated pulmonary amyloidosis: a rare clinical and radiographic entity. Chest. 2018;154(4):662A.
doi - Gandham AK, Gayathri AR, Sundararajan L. Pulmonary amyloidosis: A case series. Lung India. 2019;36(3):229-232.
doi pubmed - Seo JH, Lee SW, Ahn BC, Lee J. Pulmonary amyloidosis mimicking multiple metastatic lesions on F-18 FDG PET/CT. Lung Cancer. 2010;67(3):376-379.
doi pubmed - Guasch M, Ojanguren A, Gomez JR. Pulmonary amyloidosis: A diagnostic challenge. Cir Esp (Engl Ed). 2020;98(1):50-52.
doi pubmed - Standaert C, Herpels V, Seynaeve P. A Solitary Pulmonary Nodule: Pulmonary Amyloidosis. J Belg Soc Radiol. 2018;102(1):20.
doi pubmed - Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92(5):1325-1330.
doi pubmed - Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, Masters CL, et al. A primer of amyloid nomenclature. Amyloid. 2007;14(3):179-183.
doi pubmed - Westermark P, Bergstrom J, Solomon A, Murphy C, Sletten K. Transthyretin-derived senile systemic amyloidosis: clinicopathologic and structural considerations. Amyloid. 2003;10(Suppl 1):48-54.
doi pubmed - Peng X, Wang X, Luo D, Zuo W, Yao H, Zhang W. Atypical primary pulmonary amyloidosis: A rare case report. Medicine (Baltimore). 2020;99(26):e20828.
doi pubmed - Vieira IG, Marchiori E, Zanetti G, Cabral RF, Takayassu TC, Spilberg G, Batista RR. Pulmonary amyloidosis with calcified nodules and masses - a six-year computed tomography follow-up: a case report. Cases J. 2009;2:6540.
doi pubmed - Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann Intern Med. 1996;124(4):407-413.
doi pubmed - Al-Umairi RS, Al-Lawati F, Al-Busaidi FM. Nodular pulmonary amyloidosis mimicking metastatic pulmonary nodules: a case report and review of the literature. Sultan Qaboos Univ Med J. 2018;18(3):e393-e396.
doi pubmed - Milani P, Basset M, Russo F, Foli A, Palladini G, Merlini G. The lung in amyloidosis. Eur Respir Rev. 2017;26(145):170046.
doi pubmed - Gomez Correa GA, Osorno Serna J, Caceres Acosta MF, Caceres Gonzalez JD, Calle Ramirez JA, Sandoval Mesa JP, Roldan Perez MI. Nodular pulmonary amyloidosis: a manifestation of Sjogren's syndrome. Case Rep Pulmonol. 2018;2018:9745935.
doi pubmed - Lachmann HJ, Hawkins PN. Amyloidosis and the lung. Chron Respir Dis. 2006;3(4):203-214.
doi pubmed - Kumaran R, Saleh A, Amin B, Raoof S. A 73-year-old woman with mild shortness of breath and multiple central calcified pulmonary nodules. Chest. 2008;134(2):460-464.
doi pubmed - Quan XQ, Yin TJ, Zhang CT, Liu J, Qiao LF, Ke CS. (18)F-FDG PET/CT in patients with nodular pulmonary amyloidosis: case report and literature review. Case Rep Oncol. 2014;7(3):789-798.
doi pubmed - Mahmood S, Bridoux F, Venner CP, Sachchithanantham S, Gilbertson JA, Rowczenio D, Wagner T, et al. Natural history and outcomes in localised immunoglobulin light-chain amyloidosis: a long-term observational study. Lancet Haematol. 2015;2(6):e241-250.
doi pubmed - Justiz Vaillant AA, Stang CM. Lymphoproliferative Disorders. In: StatPearls. Treasure Island (FL), 2022.
- Ben-Ezra J. B-cell lymphoproliferative disorders. Hematol Oncol Clin North Am. 2002;16(2):321-337.
doi pubmed - Core JM, Alsaad AA, Jiang L, Patel NM. Nodular pulmonary amyloidosis: a complex disease with malignancy association. BMJ Case Rep. 2017;2017:bcr2017220428.
doi pubmed - Xiang H, Wu Z, Wang Z, Yao H. Nodular pulmonary amyloidosis and obvious ossification due to primary pulmonary MALT lymphoma with extensive plasmacytic differentiation: Report of a rare case and review of the literature. Int J Clin Exp Pathol. 2015;8(6):7482-7487.
- Filippi N, Diotti C, Donghi SM, Galetta D, Sedda G, Sandri A, De Camilli E, et al. Diagnostic and therapeutic implications of pulmonary lymphoma associated with nodular amyloidosis. Ann Thorac Surg. 2019;107(5):e325-e327.
doi pubmed - Moriyama E, Yokose T, Kodama T, Matsuno Y, Hojo F, Takahashi K, Nagai K, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue in the thymus of a patient with pulmonary amyloid nodules. Jpn J Clin Oncol. 2000;30(8):349-353.
doi pubmed - Lantuejoul S, Moulai N, Quetant S, Brichon PY, Brambilla C, Brambilla E, Ferretti GR. Unusual cystic presentation of pulmonary nodular amyloidosis associated with MALT-type lymphoma. Eur Respir J. 2007;30(3):589-592.
doi pubmed - Davis CJ, Butchart EG, Gibbs AR. Nodular pulmonary amyloidosis occurring in association with pulmonary lymphoma. Thorax. 1991;46(3):217-218.
doi pubmed - Wieker K, Rocken C, Koenigsmann M, Roessner A, Franke A. Pulmonary low-grade MALT-lymphoma associated with localized pulmonary amyloidosis. A case report. Amyloid. 2002;9(3):190-193.
doi pubmed - Kawashima T, Nishimura H, Akiyama H, Hirai K, Yamagishi S, Okada D, Kinoshita H, et al. Primary pulmonary mucosa-associated lymphoid tissue lymphoma combined with idiopathic thrombocytopenic purpura and amyloidoma in the lung. J Nippon Med Sch. 2005;72(6):370-374.
doi pubmed - Ihling C, Weirich G, Gaa A, Schaefer HE. Amyloid tumors of the lung—an immunocytoma? Pathol Res Pract. 1996;192(5):446-452.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


