| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 11, November 2022, pages 530-535
Pembrolizumab-Induced Myasthenia Gravis and Myositis: Literature Review on Neurological Toxicities of Programmed Death Protein 1 Inhibitors
Alfred Schwaba, Marc Assaadb, c, Rachelle Hamadib, Juda Zurndorfera, Racha Abi Melhemb, Jennifer Holtzbacha, Jeffrey Loefflerb, Muhammad Ibrahima
aDivision of Neurocritical Care, Department of Neurology, Staten Island University Hospital, Staten Island, NY 10305, USA
bDepartment of Medicine, Staten Island University Hospital, Staten Island, NY 10305, USA
cCorresponding Author: Marc Assaad, Department of Medicine, Staten Island University Hospital, Staten Island, NY 10305, USA
Manuscript submitted September 13, 2022, accepted November 8, 2022, published online November 27, 2022
Short title: Pembrolizumab-Induced MG and Myositis
doi: https://doi.org/10.14740/jmc4008
| Abstract | ▴Top |
We present herein the case of an elderly male patient, who was receiving immunotherapy for his urothelial cancer and who presented to our facility with lower extremity weakness. The patient was diagnosed with myasthenia gravis, thyroiditis, myositis and myocarditis, which were considered as immune adverse events of pembrolizumab therapy. The patient received pyridostigmine, intravenous immunoglobulin, plasmapheresis, corticosteroids, and rituximab with mild improvement of his symptoms. The patient had some neurological recovery, was discharged to a nursing facility, however he was ventilator dependent. Of importance, our case is followed by review and discussion of the literature related to immunotherapy and its side effects.
Keywords: Pembrolizumab; Monoclonal antibody; Immunotherapy; Myasthenia gravis; Neurotoxicity; Monoclonal antibody; Immune checkpoint inhibitor; Immune-related adverse events
| Introduction | ▴Top |
Pembrolizumab (Keytruda) is a monoclonal antibody that targets programmed death protein 1 (PD-1) and is considered an immune checkpoint inhibitor (ICI). It is widely used in many solid organ tumors including but not limited to non-small cell lung carcinoma, urothelial carcinoma, renal cell carcinoma and melanoma [1]. PD-1 is expressed on activated T lymphocytes that links usually to programmed death ligand 1 (PDL-1), which is excessively expressed by tumoral cells in order to evade the immune system. Pembrolizumab blocks this interaction by blocking PD-1 receptor, thus activating the immune system against malignant cells [2, 3]. The inhibition of the immune checkpoint PD-1/PDL-1 results in upregulation or enhancement of cytotoxic T lymphocyte activity against cancer [1]. This is how Keytruda, along with other immunotherapeutic agents, has changed the course of metastatic diseases and has shown favorable results in advanced stages of the illness [4].
However, there is the risk of activated T lymphocytes attacking normal tissue and leading to autoimmune toxicity [4]. Immunotherapy is very well known to cause immune-related adverse events (irAEs) that are very well described, staged, and managed by many oncological societies [5]. Severity of the irAEs is graded from 1 to 5 from mild symptoms to death respectively. Manifestations of autoimmune reactions vary from mild, such as skin rashes to severe, including Steven-Johnson syndrome with mucosal involvement. Patients often present with diarrhea, hypo- or hyperthyroidism, hypopituitarism, adrenal failure and pneumonitis [1, 2, 5, 6]. These autoimmune syndromes or irAEs are usually dose-dependent [5] and cease with early diagnosis and discontinuation of the offending agent. Neurological toxicities, while rare, occurring in 5% of patients receiving PD-1 inhibitor, can cause rapid clinical deterioration and be potentially fatal [4, 7, 8]. Furthermore, the incidence of such severe irAEs (grade 3) in patients is upwards of 15% [4, 5].
In the following case, we present a known but rare complication of pembrolizumab in a patient with bladder neoplasia who presents with immune-mediated myositis, myocarditis, thyroiditis, and myasthenia gravis (MG) with bulbar involvement leading to respiratory failure and invasive ventilation.
| Case Report | ▴Top |
Investigations
This is a case of an 81-year-old male with a past medical history of hypertension, hypothyroidism, benign prostatic hyperplasia, and an extensive smoking history of 65 pack- year, presenting to our facility for evaluation of progressive bilateral lower extremity weakness and pain. The patient had been diagnosed the previous year with stage III bladder cancer and underwent a cystectomy and subsequently treated with adjuvant immunotherapy with pembrolizumab every 3 weeks, and have completed two cycles. In the emergency department, the patient’s vital signs were stable, and his neurological exam was remarkable for decreased motor strength in bilateral lower extremities, graded as 3/5. The laboratory workup was evident for a creatine phosphokinase (CPK) of 6,500 U/L, elevated troponin level initially at 1.43 ng/mL, mild transaminitis, and acute kidney injury with a creatinine of 1.7 mg/dL. The patient was admitted for treatment of rhabdomyolysis. The patient did not exhibit any cardiac symptomatology, his electrocardiogram (EKG) findings were not suggestive of acute coronary syndrome, and as such, medical management was recommended by the cardiology team. Patient’s lower extremity weakness did not improve which has prompt the neurology evaluation for concern of myositis and empiric prednisone was initiated. A non-contrast computed tomography (CT) of the brain was obtained which displayed an old right thalamic infarct, and the magnetic resonance imaging (MRI) of the cervical and lumbar spine revealed degenerative changes, without evidence of cord compression. However, the MRI did show diffuse paraspinal soft tissue edema reflective of myositis. Along with these findings, MRI of bilateral lower extremities revealed diffuse edema in the right adductor, rectus femoris, vastus medialis, vastus intermedius and vastus lateralis muscles. Additional milder degree of muscle edema was noted in the greater saphenous and semitendinosus. Findings were suggestive of myositis. Cardiac enzymes kept increasing before stabilizing, rising the suspicion of myocarditis. Transthoracic echocardiogram (TTE) did not show any wall motion abnormality and estimated left ventricular ejection fraction (LVEF) was > 60%. Thyroid-stimulating hormone was 28.25 µIU/mL along with low free thyroxine level of 0.6 ng/dL. Further laboratory results are detailed in Table 1.
 Click to view | Table 1. Laboratory Findings |
Diagnosis
Within 2 weeks of his hospitalization, the patient developed dysphagia, diplopia, and a significant left eyelid droop, minimally responsive to the ice pack test, along with bilateral upper extremity weakness. As the patient’s respiratory status deteriorated, he was intubated for impending respiratory failure and upgraded to the neurocritical care unit for treatment of MG crisis. At that time, the patient’s negative inspiratory force (NIF) was -18 cmH2O, and his vital capacity was 850 mL. CT of the chest failed to reveal any thymoma. The MG serological workup was all negative including acetylcholine receptor (AChR) antibody, muscle-specific kinase (MuSK) antibody, lipoprotein receptor-related protein 4 (LRP4) antibody, and voltage-gated calcium channel (VGCC) antibody (Table 1). Furthermore, the patients’ myo-marker and paraneoplastic marker were negative as well. Nerve conduction studies revealed low motor amplitude suggestive of a neuromuscular junction (NMJ) disorder as shown in Figure 1. The pathology of his left thigh muscular biopsy revealed severe acute myonecrosis suggestive of an immune-mediated process. Patient was diagnosed with pembrolizumab-induced MG (iMG), myositis with concomitant myocarditis and thyroiditis.
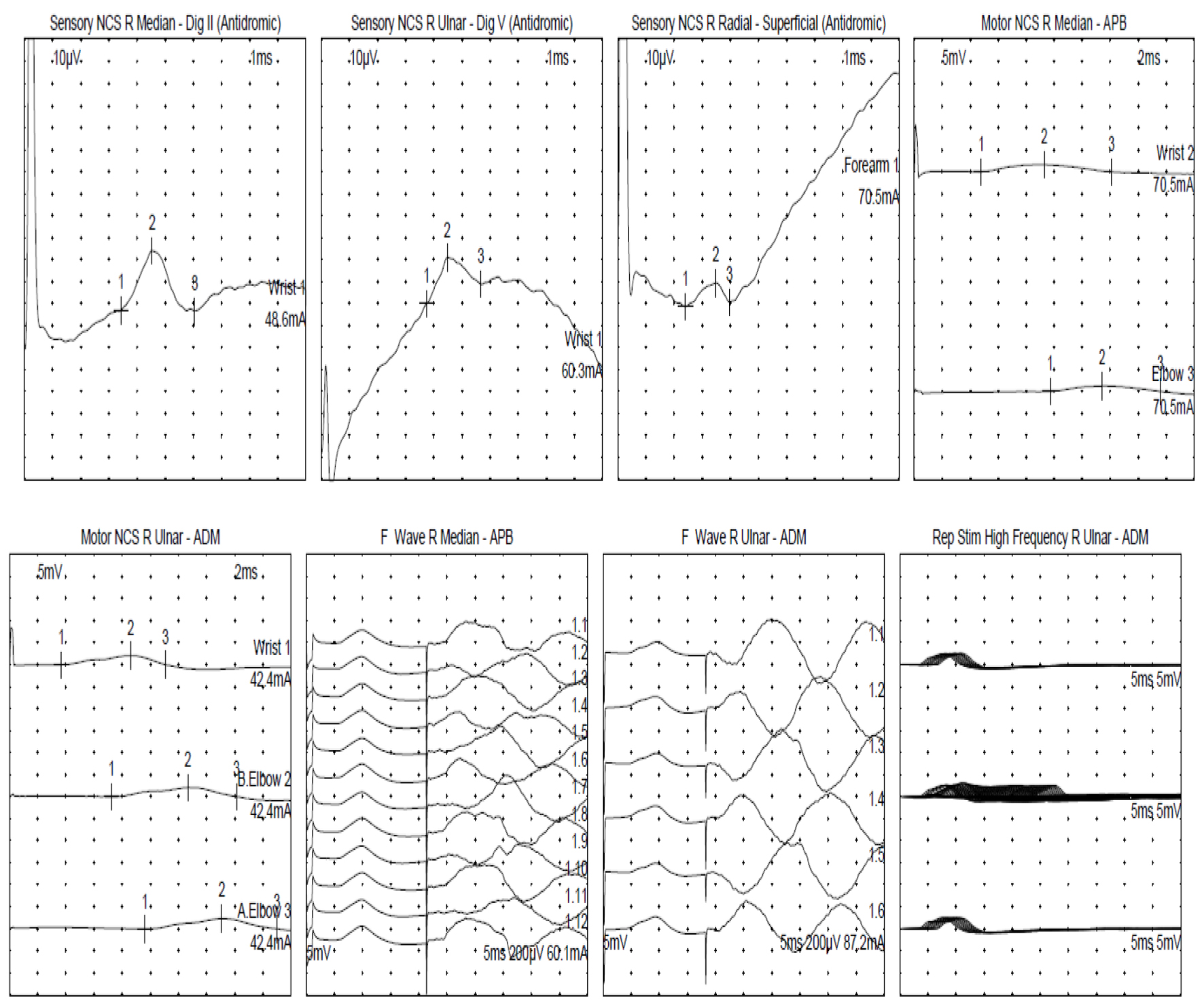 Click for large image | Figure 1. Nerve conduction study. F: frequency; ms: millisecond; mV: millivolt; NCS: nerve conduction study; R: right; Rep: repetitive; Stim: stimulation. |
Treatment
Treatment with pyridostigmine 60 mg three times daily and high-dose intravenous methylprednisolone of 1 g daily was initiated. Despite corticosteroids, the neurological status of the patient did not improve, prompting the initiation of intravenous immunoglobulin (IVIG). The patient was also receiving thyroid replacement therapy with higher dose of levothyroxine 150 µg daily. After the failure of the above-mentioned therapy, patient underwent plasmapheresis at day 6 from intensive care unit admission. Due to his inability to wean from the ventilator and prolonged intubation, tracheostomy, and a percutaneous endoscopic gastrostomy (PEG) were performed. One gram infusion of rituximab (anti-CD20) was initiated, and intravenous methylprednisolone was switched to 1 mg/kg of prednisone orally and slowly tapered over the next 4 weeks.
Follow-up and outcomes
After 4 weeks of hospitalization, the patient’s neurological symptoms started to improve and the patient was able to move bilateral lower extremities, but he did not regain his baseline motor function. The patient was discharged to a nursing facility after achieving medical stability.
| Discussion | ▴Top |
To summarize, our 81-year-old male patient was receiving Keytruda treatment for bladder neoplasia. Within 2 months of initiation of therapy, patient presented to the hospital complaining of lower extremity weakness which worsened during the hospital course and was accompanied with new onset myasthenia like syndrome that manifested as ptosis, diplopia, and dysphagia. The patient has required invasive ventilation because of his respiratory failure. Patient was diagnosed with pembrolizumab autoimmune syndrome that includes MG, myositis, thyroiditis, and myocarditis. Patient was treated with IVIG, plasmapheresis, high-dose steroid, pyridostigmine and rituximab. Towards the end of his hospital course, his motor power improved but still was ventilator-dependent via a tracheostomy.
As mentioned previously, neurotoxicity from PD-1 inhibitors is rare; however, there are many reported neurological complications including encephalitis, meningitis, cranial neuropathy, multiple sclerosis, Guillain-Barre syndrome, MG and transverse myelitis [4, 8, 9]. These complications may affect both the central and the peripheral nervous system [10, 11] and appear within 3 months of therapy initiation [12]. Clinical presentations include but are not limited to change in mental status, seizure, weakness and focal deficit [4]. Reynolds et al have listed a screening tool for each of ICI-mediated neurotoxicity along with appropriate treatment based on irAEs grade [12]. The incidence of MG as a consequence of PD-1 antagonist initiation is 30-37% according to a case series analysis [1]. Further literature review revealed that there have been similar presentations to our patient’s clinical course including those with bladder cancer who developed myositis, MG and myocarditis [7, 13-18]. The evidence highlights the possibility of certain relation between the type of malignancy and the organ or system affected by ICI, especially since iMG has been associated with concurrent myocarditis and myositis in 8% in similar cases [4, 19].
The median time of iMG onset from starting ICI is usually 1 month and the most common presenting symptom is ptosis [19]. In our case, MG autoantibodies (AchR and MuSK antibodies) were negative as well as the paraneoplastic workup and the antinuclear antibody (ANA) were all negative. While AchR antibodies are often negative in ICI-mediated MG, and the diagnosis made clinically, there have been cases with positive serological markers [4, 11-13]. An association between similar reactions and human leukocyte antigen (HLA) A02, HLA-B08 and HLA-DRB1*03 is described [15] which might be a helpful screening tool before the initiation of ICI.
The ICI-mediated MG often presents with bulbar involvement leading to respiratory failure within 1 week requiring invasive ventilation in up to 50% of patients [4, 19]. Those with elevated CPK tend to develop respiratory compromise more than those without rhabdomyolysis according to Safa et al [19]. The complete resolution of iMG symptoms and deficits is uncommon and as such, the sequalae of the disease process persist [1, 4]. Crusz at al found that the mortality rate of ICI-mediated MG is 30% [7].
Early diagnosis of neurotoxicity and iMG is crucial for the improvement of clinical outcome because the discontinuation of the immunotherapy is the important initial step [1]. In iMG as in MG, recommended therapeutic regimens include plasmapheresis, IVIG, high-dose steroids for acute symptomatic disease, as well as pyridostigmine which also has a clinical benefit [1]. Many experts suggest that high-dose methylprednisolone (1 g) is the first line agent and plasmapheresis is indicated when corticotherapy fails [1, 13]. Such doses are needed to overcome to the blood-brain barrier and to achieve better central nervous system penetration [1]. Other experts report a better outcome with the initiation of IVIG and plasmapheresis regardless of MG severity and prefer these modalities over steroids and other agents because the latter need time to achieve immunosuppression [19]. In our case, rituximab was used as a steroid tapering agent and it is also recommended by Crusz et al for a patient with a similar condition [7]. The use of other agents is also reported such as anti-tumor necrosis factor alpha (TNF-α) and cyclosporin [1]. The discontinuation of ICI in severe adverse events is recommended, and future use or resumption of these agents should be cautiously monitored. If the grade of severity of irAEs is below or equal to 3, and autoimmune symptoms have quickly resolved, it is possible to resume same ICI regimen [12].
Despite the deleterious effect of ICI, it is well known that the development of irAEs is indicative of therapeutic response. Survivors among patients with iMG or neurotoxicity related to irAEs had favorable outcome and higher tumor responsiveness [1, 19].
We describe in our case, a rare but severe complication of immunotherapy. Oncologists and internists should be aware of these manifestations to stop the medication as soon as possible, which remains the major key for recovery. Different immunosuppressive modalities are used to treat the auto-immune process; however, neurotoxicity can be fatal or irreversible.
Learning points
Neurological toxicity from PD-1 inhibitor, such as MG, is rare, however can be fatal and can have long-term sequalae. MG caused by pembrolizumab, might be irreversible and has a high mortality rate. Autoimmune antibodies in this case may be negative and the diagnosis remains clinical. Early diagnosis, early discontinuation of the culprit medication and early treatment with immunosuppressant are the key to improving prognosis. Plasmapheresis and IVIG are preferred over immunosuppressive treatment earlier in the disease course. Rituximab is a drug to consider in patients with pembrolizumab-induced MG and might have a potential benefit in other immune-mediated neurological complications.
Acknowledgments
We would like to thank the Internal Medicine Research Department and the Department of Neurology, Division of Neuro-Critical Care at Staten Island University Hospital for helping us finalize the project.
Financial Disclosure
This paper received no financial support from any third party.
Conflict of Interest
The authors declare that there is no conflict of interest.
Informed Consent
Informed consent has been obtained.
Author Contributions
All authors have made contributions to writing this case report. Marc Assaad, Rachelle Hamadi, Juda Zurndorfer, Racha Abi Melhem, Jeffrey Loeffler and Jennifer Holtzbach helped drafting the manuscript. Juda Zurndorfer and Jennifer Holtzbach participated in data collection. Alfred Schwab and Muhammad Ibrahim are responsible for the concept, editing and supervision.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Feng S, Coward J, McCaffrey E, Coucher J, Kalokerinos P, O'Byrne K. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol. 2017;12(11):1626-1635.
doi pubmed - Sonpavde GP, Grivas P, Lin Y, Hennessy D, Hunt JD. Immune-related adverse events with PD-1 versus PD-L1 inhibitors: a meta-analysis of 8730 patients from clinical trials. Future Oncol. 2021;17(19):2545-2558.
doi pubmed - Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12(11):2777-2789.
doi pubmed - Haugh AM, Probasco JC, Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf. 2020;19(4):479-488.
doi pubmed - Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-Related Adverse Events (irAEs): diagnosis, management, and clinical pearls. Curr Oncol Rep. 2020;22(4):39.
doi pubmed - Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
doi pubmed - Crusz SM, Radunovic A, Shepherd S, Shah S, Newey V, Phillips M, Lim L, et al. Rituximab in the treatment of pembrolizumab-induced myasthenia gravis. Eur J Cancer. 2018;102:49-51.
doi pubmed - Gonzalez NL, Puwanant A, Lu A, Marks SM, Zivkovic SA. Myasthenia triggered by immune checkpoint inhibitors: New case and literature review. Neuromuscul Disord. 2017;27(3):266-268.
doi pubmed - Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, Schmidgen MI, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210-225.
doi pubmed - Mohn N, Beutel G, Gutzmer R, Ivanyi P, Satzger I, Skripuletz T. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy-review of the literature and future outlook. J Clin Med. 2019;8(11):1-15.
doi pubmed - Liewluck T, Kao JC, Mauermann ML. PD-1 Inhibitor-associated Myopathies: Emerging Immune-mediated Myopathies. J Immunother. 2018;41(4):208-211.
doi pubmed - Reynolds KL, Guidon AC. Diagnosis and management of immune checkpoint inhibitor-associated neurologic toxicity: illustrative case and review of the literature. Oncologist. 2019;24(4):435-443.
doi pubmed - Takai M, Kato D, Iinuma K, Maekawa YM, Nakane K, Tsuchiya T, Yokoi S, et al. Simultaneous pembrolizumab-induced myasthenia gravis and myocarditis in a patient with metastatic bladder cancer: A case report. Urol Case Rep. 2020;31:101145.
doi pubmed - Hayakawa N, Kikuchi E, Suzuki S, Oya M. Myasthenia gravis with myositis induced by pembrolizumab therapy in a patient with metastatic urothelial carcinoma. Int Cancer Conf J. 2020;9(3):123-126.
doi pubmed - Botta C, Agostino RM, Dattola V, Cianci V, Calandruccio ND, Bianco G, Mafodda A, et al. Myositis/myasthenia after pembrolizumab in a bladder cancer patient with an autoimmunity-associated HLA: immune-biological evaluation and case report. Int J Mol Sci. 2021;22(12):1-8.
doi pubmed - Todo M, Kaneko G, Shirotake S, Shimada Y, Nakano S, Okabe T, Ishikawa S, et al. Pembrolizumab-induced myasthenia gravis with myositis and presumable myocarditis in a patient with bladder cancer. IJU Case Rep. 2020;3(1):17-20.
doi pubmed - Tian CY, Ou YH, Chang SL, Lin CM. Pembrolizumab-induced myasthenia gravis-like disorder, ocular myositis, and hepatitis: a case report. J Med Case Rep. 2021;15(1):244.
doi pubmed - Alnahhas I, Wong J. A case of new-onset antibody-positive myasthenia gravis in a patient treated with pembrolizumab for melanoma. Muscle Nerve. 2017;55(6):E25-E26.
doi pubmed - Safa H, Johnson DH, Trinh VA, Rodgers TE, Lin H, Suarez-Almazor ME, Fa'ak F, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. 2019;7(1):319.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


