| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 5, May 2022, pages 212-218
Clostridium tertium Bacteremia: A Marker of an Underlying Perforated Colonic Diverticular Disease in a Non-Neutropenic Patient With COVID-19
Eltaib Saada, d, Goar Egoryana, Shanmugha Vigneshwar Padmanabhana, Angkawipa Trongtorsaka, Akshaya Ramachandrana, Qishuo Zhanga, Khalid Mohameda, Harvey J. Friedmanb, c
aDepartment of Internal Medicine, Saint Francis Presence Hospital, Evanston, IL, USA
bDepartment of Pulmonology and Critical Care, Saint Francis Presence Hospital, Evanston, IL, USA
cDepartment of Internal Medicine, College of Medicine, the University of Illinois at Chicago (UIC), Chicago, IL, USA
dCorresponding Author: Eltiab Saad, Department of Internal Medicine, Saint Francis Presence Hospital, Evanston, IL 60202, USA
Manuscript submitted February 12, 2022, accepted March 22, 2022, published online April 12, 2022
Short title: C. tertium Infection in a COVID-19 Patient
doi: https://doi.org/10.14740/jmc3916
| Abstract | ▴Top |
Clostridium tertium (C. tertium) is an aero-tolerant, gram-positive, endospore-forming, and non-exotoxin-producing bacillus that has colonized the gastrointestinal tract of animals and humans. It is considered a rare pathogen of humans, possibly because of its low virulence. Most C. tertium infections in the reviewed literatures were predominately reported among neutropenic hosts with hematological malignancies. A 66-year-old female patient with a past medical history of type II diabetes mellitus and chronic obstructive pulmonary disease was admitted with coronavirus disease 2019 (COVID-19) that initially required non-invasive ventilation. The patient developed septic shock due to C. tertium bacteremia. Computed tomography of the abdomen depicted free intraperitoneal gas and sigmoid colon perforation. Exploratory laparotomy revealed perforated sigmoid diverticulitis, and Hartmann’s procedure was performed. The patient received a prolonged course of susceptibility-guided antibiotics to clear C. tertium bacteremia. The authors described a rare case of C. tertium bacteremia as a marker of underlying perforated colonic diverticulitis in a non-neutropenic patient with COVID-19 that necessitated operative procedure intervention for primary source control and an extended course of targeted antibiotic therapy to treat the Clostridial infection. Our case reaffirmed the available literature that suggested the presence of C. tertium bacteremia in non-neutropenic patients raises suspicion of an associated gastrointestinal tract pathology that should warrant a diagnostic workup to identify the infection source culprit.
Keywords: Clostridium tertium; Complicated diverticular disease; Colon perforation; Rare association
| Introduction | ▴Top |
Clostridium tertium (C. tertium) is a ubiquitous, gram-positive, and endospore-forming bacillus. The normal habitat of C. tertium is the soil but it has colonized the gastrointestinal tract of humans and other animals as a commensal organism [1-3]. In contrast to other Clostridia species, C. tertium does not produce exotoxins, and hence it is considered a low virulent organism that rarely causes infections in healthy humans [1-3]. Most C. tertium infections in the literature were chiefly described among neutropenic hosts with hematological malignancies [1-3], followed by only a handful of non-neutropenic patients with liver cirrhosis [4, 5] and intestinal mucosal damage due to various etiologies [2, 6, 7]. Herein, we reported an unusual case of C. tertium bacteremia as a diagnostic culprit of underlying perforated diverticulitis in a non-neutropenic patient with moderately severe coronavirus disease 2019 (COVID-19).
| Case Report | ▴Top |
Investigations
A 66-year-old female with a past medical history of type II diabetes mellitus, chronic obstructive pulmonary disease, severe pulmonary hypertension, and schizophrenia was brought to the emergency department with fatigue and shortness of breath. She denied fevers or rigors, changes in quality or color of sputum, chest pain, or palpitations, but she reported dizziness. The patient endorsed one episode of black stool overnight, but she denied any history of acute epigastric pain, lower abdominal pain, rectal bleeding, or recent bowel habits changes. The review of systems was not pertinent for any other positive symptoms. On initial evaluation, she appeared confused and ill-looking, with an oxygen saturation of 81% on room air that improved to 95% on 4 L/min oxygen. She was afebrile with a blood pressure of 110/60 mm Hg and pulse rate of 100 beats/min. There were reduced breathing sounds over both lung fields with crackles and scattered wheezes. The systemic examination was unremarkable. The initial laboratory results are summarized in Table 1. Nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was positive using polymerase chain reaction (PCR). The patient was commenced on dexamethasone 6 mg injection daily and remdesivir as per institution-based guidelines for COVID-19. The patient passed a large amount of melena and became hypotensive to 90/65 mm Hg; she responded to 1 L of intravenous fluids bolus and was transferred to the intensive care unit for close hemodynamic monitoring and non-invasive ventilation. Gastroenterology services recommended pantoprazole injections of 40 mg twice daily and an esophagogastroscopy (EGD) which revealed a bleeding duodenal ulcer that was controlled by epinephrine injection and bipolar cauterization.
 Click to view | Table 1. Pertinent Laboratory Results on Admission |
On the third day of admission, the patient spiked a high-grade fever (38.5 °C) and became hypotensive to 80/55 mm Hg and tachycardic to 140 beats/min requiring vasopressors support. Blood cultures were obtained. Physical examination was positive for inspiratory crackles over the left lower lung zone and a vague generalized abdominal tenderness. Further evaluation was limited by the patient’s altered mental status. Chest X-rays revealed consolidation of the left lower lung lobe concerning for superimposed bacterial infection. She was commenced on empiric broad-spectrum antibiotics (cefepime) per hospital-based local susceptibility patterns pending blood cultures results.
Diagnosis
Two bottles of blood cultures obtained from day 3 of admission grew out anaerobic gram-positive bacillus on day 1 of incubation. Further identification on day 2 using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry isolated C. tertium. Following Clostridia isolation on day 4 of admission, intravenous vancomycin and clindamycin were added by the infectious disease (ID) team awaiting susceptibility results. A computed tomography (CT) of the abdomen was performed to rule out a gastrointestinal infectious source in the setting of C. tertium bacteremia. The imaging depicted extensive free intraperitoneal gas (Fig. 1a) and thickened distal sigmoid colon wall with adjacent free fluids concerning for colonic perforation (Fig. 1b). There was no evidence of mesenteric ischemia.
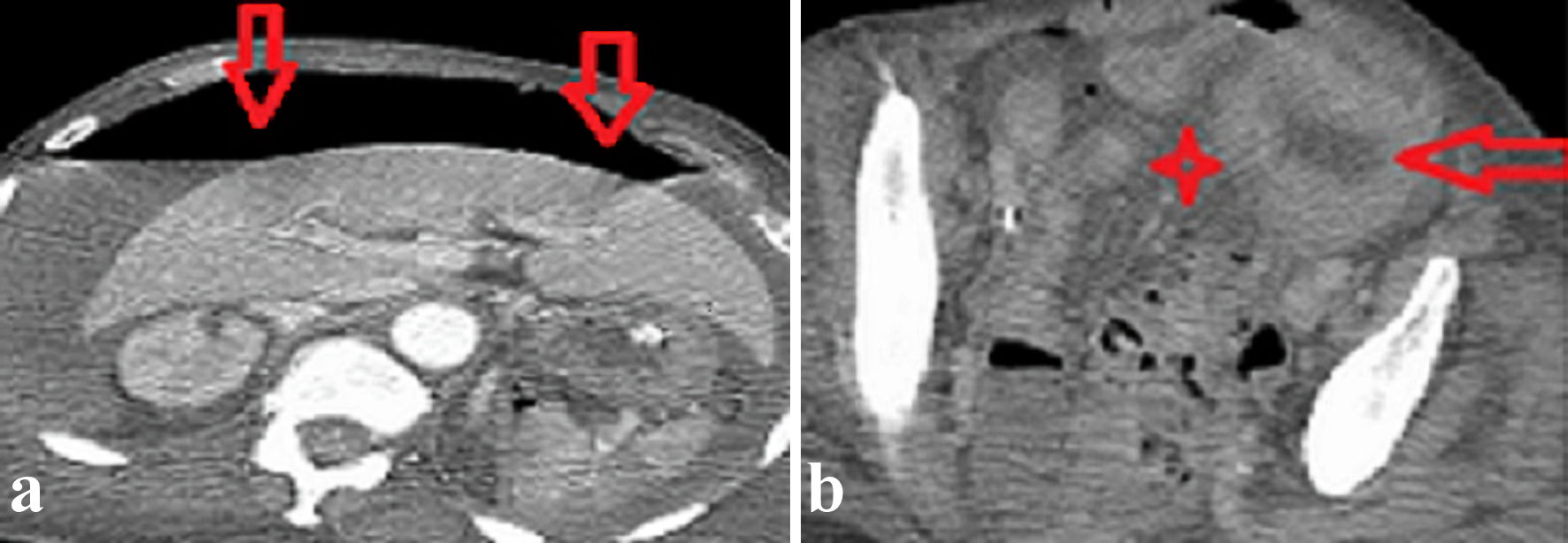 Click for large image | Figure 1. Axial images of a contrast-enhanced abdomen and pelvis CT scan revealed (a) extensive free intraperitoneal gas (vertical red arrows) and (b) a thickened sigmoid colon wall (horizontal red arrow) and focal pelvic fluids collection (red star). CT: computed tomography. |
Treatment
Surgical consultation recommended an emergent laparotomy for primary sepsis source control. Operative intervention was performed on day 4 of admission that revealed perforated sigmoid diverticulitis with localized peritonitis, and Hartmann’s procedure was subsequently performed. The patient remained on vasopressors support for 5 days postoperatively, and repeat blood cultures on day 3 and day 5 postoperatively continued to grow C. tertium. Most isolates were susceptible to meropenem, metronidazole, and amoxicillin-clavulanate, and piperacillin-tazobactam. The patient was switched to intravenous meropenem and metronidazole per susceptibility results. Histology of the resected colon biopsy confirmed perforated diverticulitis without evidence of neoplasia. The patient was continued on parenteral meropenem and metronidazole per the infectious disease team’s advice, and serial blood cultures on day 10 and day 14 postoperatively confirmed clearance of C. tertium bacteremia.
Follow-up and outcomes
The patient had a challenging postoperative course over 2 weeks. The course was complicated by difficult weaning from the mechanical ventilator due to intensive care unit-acquired weakness (ICU-AW) that necessitated a transition into tracheostomy. The patient was eventually transferred into a long-term acute care facility for ongoing tracheostomy care. She was discharged on oral metronidazole and amoxicillin-clavulanate for a further 2-week course, considering the delayed clearance of C. tertium bacteremia as recommended by the infectious disease team.
| Discussion | ▴Top |
C. tertium has been increasingly reported as a human pathogen over the last three decades [1-8], raising significant concerns of this commensal as an emerging infectious organism among certain high-risk populations [1-3]. While the vast majority of C. tertium infections manifested as febrile neutropenia in immunosuppressed hosts with hematological malignancies who received chemotherapy [1-3, 7, 8], there are a handful of cases that documented C. tertium as the culprit pathogen even in non-neutropenic patients with various associated risks including end-stage liver disease (presenting as spontaneous bacterial peritonitis) [4, 5], and a variety of conditions leading to intestinal mucosal damage (such as inflammatory bowel diseases [2, 7], infectious colitis [6], paralytic ileus [9], and perforated appendicitis with peritonitis that was complicated by a pyogenic hepatic abscess [10]). Additionally, there were individual isolated cases of acute bronchopneumonia in a patient who ingested glyphosate [11] and necrotizing fasciitis in a patient with a history of non-Hodgkin lymphoma [12]. Table 2 [1-14] summarizes the clinical presentation, possible risk factors, and susceptibility results of most of the reported C. tertium cases.
 Click to view | Table 2. Summary of the Reported Cases of C. tertium Bacteremia as per Literature Review 1990 - 2022 (Including the Presented Case) |
To the best of our knowledge, this reported case would be the first one to demonstrate an association between C. tertium and perforated colonic diverticular disease. Interestingly, the isolation of C. tertium from the blood cultures was the initial clue to an underlying, perhaps serious, gastrointestinal tract perforation in our critically ill patient who exhibited minimal peritonitic signs on the physical examination, presumably due to altered sensorium related to hypoxic respiratory failure resulting from COVID-19. The injury to the colonic mucosa likely led to translocation of C. tertium into the systemic circulation causing bacteremia, as being explained in other similar cases of C. tertium that were associated with a disturbed intestinal mucosal integrity [2, 6, 7, 9, 10].
It is worth mentioning that gastrointestinal perforation has been reported in several patients with COVID-19 per a recent pandemic literature [15]. However, it remains unclear whether the COVID-19 worsens the risk of perforation of the pre-existing diverticular disease (as in our patient) or not. Additionally, this patient had received corticosteroids that may have masked the peritonitic signs and delayed the early diagnosis of acute diverticulitis. Our patient also suffered from poorly controlled diabetes which may have both compounded the risk of diverticular perforation and decreased the clearance of C. tertium bacteremia [16]. All of the above discussed risks have probably accumulatively resulted in the occurrence of the septic shock due to C. tertium bacteremia that complicated perforated diverticulitis necessitating Hartmann’s operation to control the sepsis source.
It is interesting to note that C. tertium species were reportedly difficult to isolate from routine cultures [1, 2], as these isolates are aerotolerant and only slowly growing when utilizing traditional culturing methods [1]. The latter microbiological observation might have led to underdiagnoses in the past [1, 5].
Furthermore, the identification of C. tertium species has been largely confused with Bacillus species and Lactobacillus species because of the similar culture’s growth patterns and micromorphology [5, 11], which could have resulted in inaccurate identification and subsequently inappropriate antibiotics selection [1, 17]. Nevertheless, modern bacterial identification diagnostics, such as direct MALDI-TOF mass spectrometry, which was employed in our case and other two cases [5, 13], have facilitated rapid as well as accurate isolation of C. tertium species, and therefore allowed selection of targeted antibiotic therapy based on susceptibility results, which is particularly vital in C. tertium cases, relative to the other Clostridia species, because of the reported resistance of some strains to many antibiotics, including the third and fourth generation cephalosporins [2, 3, 11]. Moreover, molecular biology techniques (such as 16S rRNA sequencing) have also been used for the fast identification of C. tertium species [11].
The pathogenesis of C. tertium remains largely unclear as this organism is non-exotoxin-producing [1-3]. It was theorized that the major four predisposing factors implicated in the pathogenesis of C. tertium were neutropenia, gastrointestinal mucosal injury with bacterial translocation, end-stage liver disease, and the recent use of broad-spectrum antibiotics that may predispose to intestinal colonization with C. tertium [1-5]. Many of the reported patients have had more than one risk factor [2], for instance, chemotherapy results in neutropenia that significantly diminishes the innate immune response to clear C. tertium bacteremia, and it also causes intestinal mucosal injury that potentiates translocation of C. tertium into the systemic circulation [1, 2]. Additionally, most neutropenic patients at the time of isolation of C. tertium had received broad-spectrum antibiotics, as empiric therapy for neutropenic fevers, which may have selectively favored intestinal colonization with C. tertium [2].
It has been recommended to treat C. tertium infections aggressively with targeted antibiotic therapy, despite being potentially a non-highly virulent organism with a relatively low direct mortality rate [1, 5]. One-month mortality rate following a blood culture isolation of C. tertium was reported to be 34% in the largest series of 32 cases with C. tertium bacteremia that was reported by Miller et al from Duke University Medical Center [2]. Such high reported mortality was largely attributed to the advanced stage of malignancies and the burden of the associated medical comorbidities rather than C. tertium infection itself [2], suggesting that the latter infection could be a marker of underlying poor baseline status.
There are limited data on the standard duration of directed antibiotic therapy in the available literature [1-3]. We employed a prolonged course of targeted antibiotics for C. tertium bacteremia clearance (i.e., 4 weeks) due to the persistence of Clostridial growth on one blood culture postoperatively, presumably attributable to the immunosuppressive status of our patient that resulted from poorly controlled diabetes and concurrent corticosteroids use.
Conclusions
The authors described a rare case of C. tertium bacteremia as a marker of underlying perforated colonic diverticulitis in a non-neutropenic patient with COVID-19 that necessitated operative intervention for primary source control and an extended course of targeted antibiotic therapy to treat the Clostridial infection. Our case reaffirmed the available literature which suggested the presence of C. tertium bacteremia in non-neutropenic patients raises suspicion of an associated gastrointestinal tract pathology that should warrant a diagnostic workup to identify the infectious culprit.
Learning points
C. tertium bacteremia in non-neutropenic patients raises suspicion of an associated gastrointestinal tract pathology that should warrant a diagnostic workup to identify the infectious culprit. Modern bacterial identification diagnostics, such as direct MALDI-TOF mass spectrometry have facilitated rapid as well as accurate isolation of C. tertium species and therefore allowed selection of targeted antibiotic therapy based on susceptibility results.
Acknowledgments
The authors would like to acknowledge the Department of Intensive Care Unit at Saint Francis Presence Hospital for providing valuable input to this case presentation.
Financial Disclosure
The authors confirm that there is no funding to declare regarding the publication of this case report.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Informed Consent
Informed written consent was obtained from the patient to write and publish her case as a case report with all accompanying clinical and radiological images.
Author Contributions
ES and GE contributed to the conceptualizing and writing the first manuscript. VP, AT, AR, QZ, and KM contributed to editing the final draft. HF performed the critical review. All authors were involved in the clinical management of the reported patient. All authors agreed to the final draft submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Shah S, Hankenson J, Pabbathi S, Greene J, Nanjappa S. Clostridium tertium in neutropenic patients: case series at a cancer institute. Int J Infect Dis. 2016;51:44-46.
doi pubmed - Miller DL, Brazer S, Murdoch D, Reller LB, Corey GR. Significance of Clostridium tertium bacteremia in neutropenic and nonneutropenic patients: review of 32 cases. Clin Infect Dis. 2001;32(6):975-978.
doi pubmed - Steyaert S, Peleman R, Vaneechoutte M, De Baere T, Claeys G, Verschraegen G. Septicemia in neutropenic patients infected with Clostridium tertium resistant to cefepime and other expanded-spectrum cephalosporins. J Clin Microbiol. 1999;37(11):3778-3779.
doi pubmed - Wazir M, Jain AG, Nadeem M, Ur Rahman A, Everett G. Clostridium tertium Bacteremia in a Non-neutropenic Patient with Liver Cirrhosis. Cureus. 2019;11(4):e4432.
doi - Sutton SS, Jumper M, Shah A, Edun B. Clostridium tertium peritonitis and concurrent bacteremia in a patient with a history of alcoholic cirrhosis. J Investig Med High Impact Case Rep. 2017;5(3):2324709617731457.
doi pubmed - Chalhoub V, Kallab R, El Hajj A, Hachem K, Yazbeck P. Septic shock due to Clostridium tertium in an immunocompetent patient following colitis without inflammatory bowel disease. Anaesth Crit Care Pain Med. 2016;35(2):167-168.
doi pubmed - Gosbell IB, Johnson CG, Newton PJ, Jelfs J. Clostridium tertium bacteremia: 2 cases and review. Pathology. 1996;28(1):70-73.
doi pubmed - Coleman N, Speirs G, Khan J, Broadbent V, Wight DG, Warren RE. Neutropenic enterocolitis associated with Clostridium tertium. J Clin Pathol. 1993;46(2):180-183.
doi pubmed - Tappe D, Dirks J, Muller R, Brederlau J, Abele-Horn M, Suerbaum S, Kurzai O. Fatal Clostridium tertium septicemia in a nonneutropenic patient. J Infect. 2005;50(1):76-80.
doi pubmed - Milano V, Biehle L, Patel S, Hammer J. Clostridium tertium bacteremia and hepatic abscess in a non-neutropenic patient. IDCases. 2019;15:e00510.
doi pubmed - You MJ, Shin GW, Lee CS. Clostridium tertium bacteremia in a patient with glyphosate ingestion. Am J Case Rep. 2015;16:4-7.
doi pubmed - Ray P, Das A, Singh K, Bhansali A, Yadav TD. Clostridium tertium in necrotizing fasciitis and gangrene. Emerg Infect Dis. 2003;9(10):1347-1348.
doi pubmed - Salvador F, Porte L, Duran L, Marcotti A, Perez J, Thompson L, Noriega LM, et al. Breakthrough bacteremia due to Clostridium tertium in a patient with neutropenic fever, and identification by MALDI-TOF mass spectrometry. Int J Infect Dis. 2013;17(11):e1062-1063.
doi pubmed - Vanderhofstadt M, Andre M, Lonchay C, Levecque P, Holemans X, Canon JL, D'Hondt L. Clostridium tertium bacteremia: contamination or true pathogen? A report of two cases and a review of the literature. Int J Infect Dis. 2010;14(Suppl 3):e335-337.
doi pubmed - Bulte JP, Postma N, Beukema M, Inberg B, Stegeman AG, van der Hoeven H. COVID 19 and the risk of gastro-intestinal perforation: A case series and literature review. J Crit Care. 2022;67:100-103.
doi pubmed - Cologne KG, Skiada D, Beale E, Inaba K, Senagore AJ, Demetriades D. Effects of diabetes mellitus in patients presenting with diverticulitis: clinical correlations and disease characteristics in more than 1,000 patients. J Trauma Acute Care Surg. 2014;76(3):704-709.
doi pubmed - Grosse-Herrenthey A, Maier T, Gessler F, Schaumann R, Bohnel H, Kostrzewa M, Kruger M. Challenging the problem of clostridial identification with matrix-assisted laser desorption and ionization-time-of-flight mass spectrometry (MALDI-TOF MS). Anaerobe. 2008;14(4):242-249.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


