| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 7, July 2013, pages 507-510
Value of Prostate-Specific Antigen Elevated in Transudative Pleural Effusion for Diagnosis of Prostate Cancer-Induced Paramalignant Pleural Effusion
Naoko Fujiwaraa, Hitoshi Sugawaraa, d, Hiroki Yabea, Akira Ishiia, Hiroshi Matsubayashia, Tamami Watanabea, Masafumi Kakeia, Yutaka Kobayashib, Yoh Dobashic
aDivision of General Medicine, The First Department of Comprehensive Medicine, Saitama Medical Center, Jichi Medical University, Saitama, Japan
bDivision of Urology, The Second Department of Comprehensive Medicine, Saitama Medical Center, Jichi Medical University, Saitama, Japan
cDepartment of Pathology, Saitama Medical Center, Jichi Medical University, Saitama, Japan
dCorresponding author: Hitoshi Sugawara, FACP, Division of General Medicine, The First Department of Comprehensive Medicine, Saitama Medical Center, Jichi Medical University, 1-847, Amanuma-cho, Omiya-ku, Saitama-city, Saitama 330-8503, Japan
Manuscript accepted for publication May 16, 2013
Short title: Prostate-Specific Antigen in Transudative Pleural Effusion
doi: https://doi.org/10.4021/jmc1322w
| Abstract | ▴Top |
When evaluating pleural effusion of undetermined etiology, malignant disease cannot be ruled out even if the effusion is transudative. Measurement of tumor markers in transudative pleural effusion (TPE) may aid in diagnosis, but the exact utility of these markers is unclear. We report the case of a 78-year-old man with paramalignant pleural effusion (PMPE) due to prostate cancer diagnosed by measuring prostate-specific antigen (PSA) in TPE. Androgen blockade therapy was effective in treating the PMPE. We discuss the value of PSA elevated in TPE for diagnosis of prostate cancer induced-PMPE.
Keywords: Prostate cancer; Prostate-specific antigen; Transudative pleural effusion; Paramalignant pleural effusion; Malignant pleural effusion
| Introduction | ▴Top |
Although pleural effusion (PE) is common, diagnosis of its precise etiology is difficult in some cases with possible malignancy, irrespective of pathology, particularly in cases of transudative pleural effusion (TPE). Because TPE is caused by malignant disease in 3-10% of cases [1], the distinction between TPE due to benign conditions or to malignancy with concomitant disease such as heart failure may be difficult.
Malignant PE is diagnosed based on the presence of malignant cells by cytology or pleural biopsy. PE in cases of known malignancy without cytological evidence of malignancy is termed paramalignant pleural effusion (PMPE) [2].
In one report, the cause of PMPE could be only established in 88 (59%) of 150 patients, major causes being lung cancer (41%) and breast cancer (13%) [3].
In diagnosis of the etiology of TPE, no single tumor marker has exceptional diagnostic accuracy [4]. The sensitivity of prostate-specific antigen (PSA) for evaluating the etiology of TPE in patient with prostate cancer is not yet known.
Here, we measured PSA in TPE of a man with prostate cancer and diagnosed prostate cancer-induced PMPE. Further, we discuss the possible mechanisms of prostate cancer-induced PMPE.
| Case Report | ▴Top |
A 78-year-old man had experienced exertional dyspnea for 1 year prior to admission. He had well-controlled hypertension. Bilateral PE was detected at a local clinic. Treatment with diuretics did not improve the dyspnea. Six months before admission, he was referred to our medical center, where thoracentesis was performed. The PE was transudative but physical examination, brain natriuretic peptide (BNP) tests, and echocardiography did not indicate heart failure. Following thoracic drainage, the dyspnea improved and he discontinued hospital visits.
One week before admission, the patient again developed exertional dyspnea and was admitted for further examination. He reported a weight loss of 10 kg over the previous year without appetite loss and had no urinary voiding symptoms. His body mass index was 19 kg/m2; body temperature, 36.5°C; blood pressure, 118/80 mmHg; heart rate, 93 beats/min (regular); and respiratory rate, 18 breaths/min. His general appearance was good. Physical findings included weakness of respiratory sounds in both lower lung fields, no heart murmur, and edema in both lower extremities. Digital rectal examination revealed an elastic hard 5-cm prostate with induration.
Laboratory tests (Table 1) revealed absence of proteinuria, slight hypoalbuminemia, slightly elevated serum creatinine and BNP levels, hypoxemia, and respiratory alkalosis.
 Click to view | Table 1. Summary of the Investigations Performed: All Positive and Relevant Negative Results are Shown |
Chest radiography (Fig. 1A) and computed tomography (Fig. 1B, C) showed changes indicative of pulmonary lymphangitis carcinomatosa. Echocardiography confirmed absence of heart failure (Table 1).
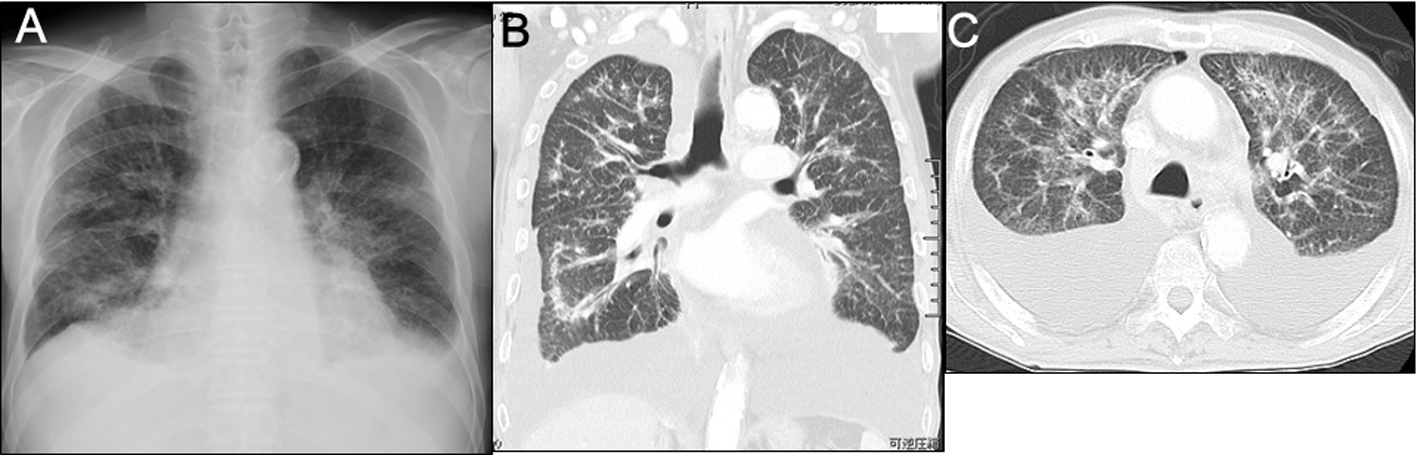 Click for large image | Figure 1. Imaging studies on admission. A) Plain chest radiography showed the cardiothoracic ratio was 55%, bilateral costophrenic angles were dull, and diffuse bilateral reticulonodular opacification was present. B, C) Plain chest computed tomography showed bilateral large amounts of pleural fluid, slight pericardial effusion, patchy consolidation sparing the subpleural regions, and thickenings of both the interlobular septum and pleura. |
Because of the indurated prostate and elevated serum PSA (166.0 ng/mL), transrectal prostate biopsy was done and revealed Gleason's grade 4 adenocarcinoma. Immunohistochemical detection of PSA confirmed the diagnosis. Bone scintigraphy showed several bone metastases in both humeri, right iliac bone, and sternum.
Because the PE protein-to-serum protein ratio was 0.49 (3.4/6.9) and the PE lactate dehydrogenase (LDH)-to-serum LDH ratio was 0.49 (101/204), < 0.6 and 0.5, respectively, it was classified as transudative [5]. Additionally, absolute PE LDH was 101 mU/mL, less than two-thirds of the normal serum LDH upper limit. Though repeated cytology of the PE did not show malignant cells, the PSA level in PE was elevated (192.0 ng/mL). Therefore, TPE was diagnosed with prostate cancer-induced PMPE.
On hospital day 17, we initiated androgen blockade therapy: 80 mg oral bicalutamide daily and 3.75 mg subcutaneous leuprorelin acetate once every week.
On hospital day 30, 2 weeks after starting treatment, the PaO2 level had increased to 72 mmHg with room air, and serum and PE PSA values had decreased to 65.0 ng/mL and 109 ng/mL, respectively. The patient was discharged on day 30.
The serum PSA level decreased to 21.6 ng/mL 30 days after discharge and to 4.4 ng/mL 120 days after discharge.
| Discussion | ▴Top |
The diagnosis of prostate cancer was clear from the physical examination and elevated PSA. But the etiology of TPE was uncertain, especially whether benign or not. According to the classical algorithmic approach for diagnosis of PE, usual TPE etiologies include congestive heart failure, liver cirrhosis, nephrotic syndrome, acute atelectasis, and congestive pericarditis.
When TPE of undetermined etiology suggests the possibility of malignant disease, PSA levels in TPE should be measured in prostatic cancer patients, even in cases without malignant cytology. Brown et al. reported a case of prostate cancer diagnosed based on elevated PSA despite negative cytology in PE, normal age-adjusted serum PSA, and no radiographic evidence of metastatic disease [6].
High PE PSA levels suggest that PMPE is due to prostate cancer. PSA, a glycoprotein expressed specifically in the cytoplasm of prostatic cells but not in other normal tissues or tumors, is the most sensitive marker of prostate cancer [7]. Cascinu measured malignant effusion PSA levels in 89 patients with primary malignancies in the colon, stomach, breast, liver, prostate, lung, and kidney tissues. PSA in PE was positive in 5 of 5 prostate cancer cases, and negative in other cancers [8].
PMPE due to prostate cancer may be from either lymphatic or hematogenous origin (Fig. 2). Lymphatic obstruction may cause TPE [9] and usually shows negative cytology. PSA production by prostate cancer with tumor-related lymphatic obstruction results in high PSA levels in TPE. In contrast, blood-borne metastases cause pulmonary parenchymal or pleural involvement and usually cause exudative PE. However, atelectasis attributed to bronchial obstruction, either due to parenchymal or pleural involvement, can raise TPE PSA levels in some cases.
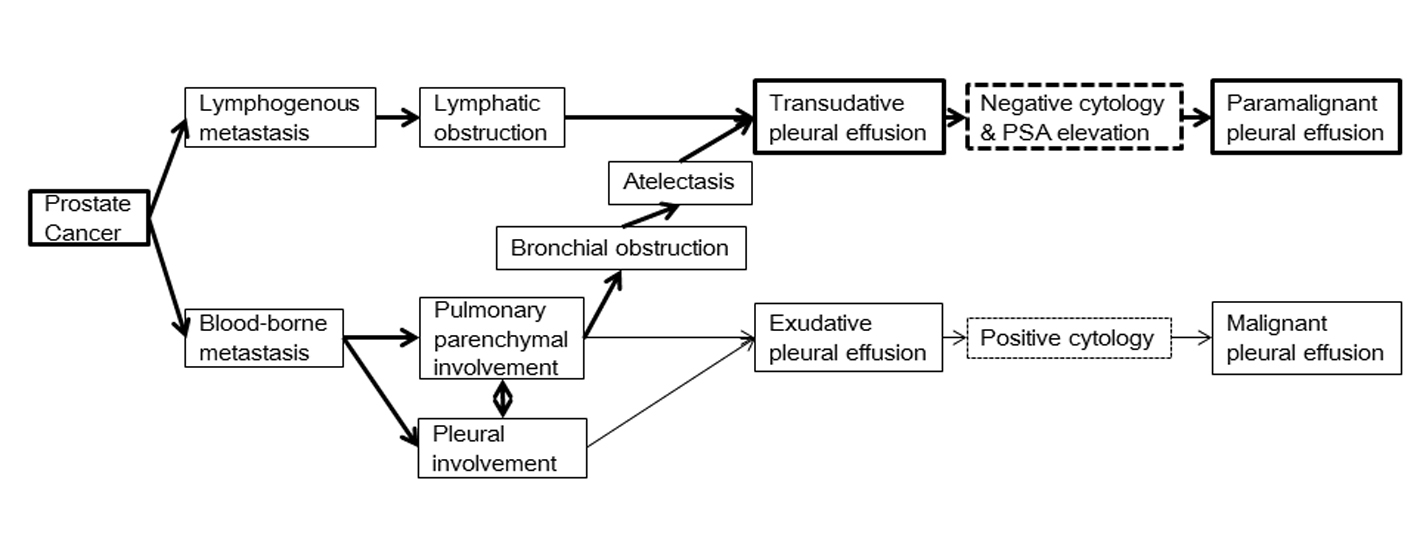 Click for large image | Figure 2. Possible mechanisms of prostate cancer-induced paramalignant pleural effusion. Lymphatic obstruction causes transudative pleural effusion (TPE), and usually exhibits negative cytology. When there is prostate tumor-related lymphatic obstruction, prostate-specific antigen (PSA) will be high in TPE. Blood-borne metastases cause pulmonary parenchymal or pleural involvement, resulting in exudative pleural effusion in most cases. Atelectasis attributed to bronchial obstruction either due to parenchymal or pleural involvement could increase PSA levels in TPE of some cases. |
Androgen blockade therapy was effective in this case. Carrascosa reported the case of a 73-year-old man with prostate cancer-induced malignant PE, which resolved with flutamide and leuprorelin acetate [10]. Intrathoracic metastasis is indicative of advanced disease with remote metastasis, which typically has no effective treatment. However, hormonal therapy is often effective for advanced prostate cancer.
In conclusion, measurement of PSA in TPE is a helpful adjunct for diagnosis when TPE is of uncertain etiology and lacks malignant cytology in patients with prostate cancer.
Acknowledgments
We thank Dr. Wilfred Y. Fujimoto for critical reading and helpful suggestions of the manuscript.
Grant Support
We have no grant support associated with this publication.
Conflict of Interest
All authors report no conflicts of interest associated with this publication and there has been no financial support for this paper that could have influenced its conclusion.
| References | ▴Top |
- Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83(2):235-250.
pubmed - Sahn SA. Pleural diseases related to metastatic malignancies. EurRespir J. 1997;10(8):1907-1913.
doi pubmed - Gurung P, Goldblatt MR, Huggins JT, Doelken P, Sahn SA. Pleural fluid characteristics of paramalignant pleural effusion. Chest 2009; 136: 44s-c-45s.
- Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang J, Yang HB. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax. 2008;63(1):35-41.
doi pubmed - Light RW, Macgregor MI, Luchsinger PC, Ball WC, Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507-513.
doi pubmed - Brown GA, Ginsberg PC, Harkaway RC. Prostatic adenocarcinoma diagnosed by prostate-specific antigen analysis of pleural fluid. Urol Int. 1998;60(3):197-198.
doi pubmed - Gittes RF. Carcinoma of the prostate. N Engl J Med. 1991;324(4):236-245.
doi pubmed - Cascinu S, Del Ferro E, Barbanti I, Ligi M, Fedeli A, Catalano G. Tumor markers in the diagnosis of malignant serous effusions. Am J ClinOncol. 1997;20(3):247-250.
doi pubmed - Heffner JE, Sahn SA, Hollingsworth H. Diagnostic evaluation of a pleural effusion in adults: Initial testing. In: UpToDate, Basow DS (Ed), UpToDate, Waltham, MA, 2013.
- Carrascosa M, Perez-Castrillon JL, Mendez MA, Cillero L, Valle R. Malignant pleural effusion from prostatic adenocarcinoma resolved with hormonal therapy. Chest. 1994;105(5):1577-1578.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


