| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 7, July 2013, pages 450-457
Neutropenia in a Patient With Rheumatoid Arthritis Associated With αβ/γδ T-Cell Large Granular Lymphocyte Lymphoproliferative Disorder Manifesting as Felty’s Syndrome: From Diagnosis to Therapy
Renata Oliveira Cabrala, d, Catarina Panelas Laua, b, Joao Rodriguesa, c, Margarida Maria de Carvalho Limaa, b
aHaematology Department, Hospital de Santo Antonio (HSA), Centro Hospitalar do Porto (CHP), Porto, Portugal
bCytometry Laboratory, Hospital de Santo Antonio (HSA), Centro Hospitalar do Porto (CHP), Porto, Portugal
cUnit of Molecular Biology, Hospital de Santo Antonio (HSA), Centro Hospitalar do Porto (CHP), Porto, Portugal
dCorresponding author: Renata Oliveira Cabral, Hospital Santo Antonio, Servico Hematologia Clinica, Largo professor Abel Salazar, Oporto, PT 4099-001, Portugal
Manuscript accepted for publication March 20, 2013
Short title: Neutropenia as Felty’s Syndrome
doi: https://doi.org/10.4021/jmc1176w
| Abstract | ▴Top |
T-cell large granular lymphocytic (LGL) leukaemias are clonal proliferations of cytotoxic T-cells that are frequently associated with rheumatoid arthritis (RA) and neutropenia and are usually controlled with immunosuppressive drugs and growth factors. We report a case of a 51-years-old patient with RA, mild splenomegaly and severe autoimmune neutropenia, who have failed to respond to methotrexate, leflunamide and corticosteroids and had a suspicion of granulocyte-colony stimulating factor (G-CSF) induced thrombocytopenia. The diagnosis of Felty’s syndrome was done and phenotypically abnormal TCR-αβ and TCRγδ T-LGL were detected in blood, although PCR-based studies provided evidence only for clonal TCRG gene rearrangements. A second attempt to control neutropenia with G-CSF induced thrombocytopenia again. Cyclosporine A was effective in controlling neutropenia, the dose being adjusted in order to maintain acceptable neutrophil counts and to minimise side effects. These included hypertension, renal insufficiency and gingival hypertrophy, the later being worsened by the concomitant use of amlodipine.
Keywords: Large granular lymphocytes; Rheumatoid arthritis; Neutropenia; Cyclosporine A; Granulocyte-Colony Stimulating Factor; Amlopidine; Thrombocytopenia; Gingival hypertrophy.
| Introduction | ▴Top |
Large granular lymphocytes (LGL) represent up to 15% of the peripheral blood lymphocytes in normal adult individuals, where most LGL are natural killer cells and only about 15% are cytotoxic T cells (CTL). However, 85% of the LGL leukaemia (LGLL) cases are derived from CTL, the majority of cases being T cell receptor (TCR)- αβ+CD8+ and a minority arising from TCR-αβ+CD4+ or TCR-γδ+ T cells [1].
T-cell derived LGL lymphoproliferative disorders (LPD) do represent a continuum spectrum of transient or persistent polyclonal, oligoclonal and monoclonal CTL proliferations and are more frequent in patients with chronic inflammatory conditions, infectious or autoimmune diseases or that underwent splenectomy or transplantation; in order to establish the diagnosis of a T-cell LGL leukaemia (T-LGLL), the expanded T-cells should be proven to be monoclonal [1-3]. T-LGLL frequently associates with cytopenias and immunological abnormalities, including dysgammaglobulinemia, autoantibodies and circulating immune complexes [1-5]. They sometimes coexist with autoimmune disorders, rheumatoid arthritis (RA) occurring in up to 30% of patients [1]. Felty’s syndrome is a clinical entity defined by the triad of RA, neutropenia, and splenomegaly [6].
Although the aetiology of T-LGL-LPD is not known, there is evidence for an antigen-driven CTL proliferation that ultimately leads to the development of a clonal LGL expansion [2-5]. Chronic T-cell activation with auto-antigens has been proposed as the initial stimulus and evidence for a viral infection was provided for specific T-LGLL subtypes [7]. Deregulation of Fas/Fas ligand (FasL) induced CTL apoptosis have also been documented [8].
The diagnosis of T-LGL-LPD (including T-LGLL) should be suspected in patients with unexplained cytopenias and increased numbers of LGL in blood, documented either by morphology and/or by flow cytometry [1]. Flow cytometry is useful not only to demonstrate increased numbers of CTL, but also to evidence an aberrant CTL immunophenotype and an expansion of specific families of the variable domain of the TCR -α, -β, -γ and -δ chains; clonal rearrangements of the TCR genes are documented by Polymerase Chain Reaction (PCR) based molecular studies [1-3].
T-cell derived LGLL are usually clinically indolent, with a median survival time of over 10 years. Neutropenia is the most significant clinical problem in patients with TCR-αβ+CD8+ and TCR-γδ+ T-LGLL (85%), severe neutropenia being present in about 45% of cases [1-3], although being rarely observed in TCR-αβ+CD4+ T-LGLL [3]. Anaemia and thrombocytopenia are found in about 48% and 20% of patients, respectively [1]. Mild to moderate splenomegaly occurs in 20-50% of cases, whereas hepatomegaly is found in only 10-20% [1].
In spite of the indolent behaviour, the majority of patients with T-LGLL require treatment at some point during the disease course and the most common indications are symptomatic cytopenias, especially chronic neutropenia associated with recurrent infections [1, 9]. Improvement of cytopenias is usually achieved with immunosuppressive agents, including methotrexate (MTX), cyclophosphamide and cyclosporine A (CsA); corticoids alone normally do not produce sustained neutrophil increase, and secondary long-term effects are not negligible; treatment with G-CSF may also improve neutropenia, being particularly beneficial for short periods of time during infectious complications [9-12].
Herein, we report a case of a patient with RA with 15 years of evolution and a proliferation of TCRαβ+ and TCR-γδ T-LGL who suffered from severe chronic neutropenia, refractory to MTX, leflunamide and corticoids. Attempts to correct the neutropenia with G-CSF during undercurrent infections resulted in thrombocytopenia for two times. Finally, CsA therapy made possible a sustained neutrophil recovery, dose adjusting being done in order to avoid secondary effects; one of them, gingival hyperplasia, was substantially worsened due to using amlodipine to control hypertension and renal insufficiency.
| Case Report | ▴Top |
A 51-year-old Portuguese Caucasian man with RA was referred to our haematology consultation in December 2009 for investigation of severe chronic neutropenia. On physical examination, he presented bilateral swelling, stiffness and deformity of the proximal interphalangeal and metacarpophalangeal joints, as well as a mild splenomegaly.
In the past, he has received several therapies. Shortly after the diagnosis of RA in 1995, he was treated with gold salts and two years later he started with oral prednisone (5 mg/day) and MTX (5 mg/week) which were maintained for about five years. In 2007, after consulting his doctor due to worsening of the osteoarticular complaints, he restarted on prednisone (10 mg/day) and MTX (10 mg/week), the later being suspended in January 2008, because of sustained neutropenia. Two months afterward, he started on oral leflunamide, which was discontinued one month later because of worsening of the neutropenia (neutrophils 0.1 × 109/L) and a serious skin infection on his hand, refractive to oral flucloxacillin. For that reason, he was hospitalized and received oral prednisolone (100 mg/day, with a rapid decrease in dosage) and subcutaneous G-CSF (300 µg/day). Neutrophil recover was observed 10 days later, reaching 7.9 × 109/L (Fig. 1A). However, because of a progressive decrease in platelet counts from 181 to 87 × 109/L, it was decided to stop G-CSF, and neutrophils declined again to 0.5 × 109/L (Fig. 1A). From 2008 to 2009, he remained on oral prednisone 5 - 10 mg/day, with persistence of asymptomatic neutropenia.
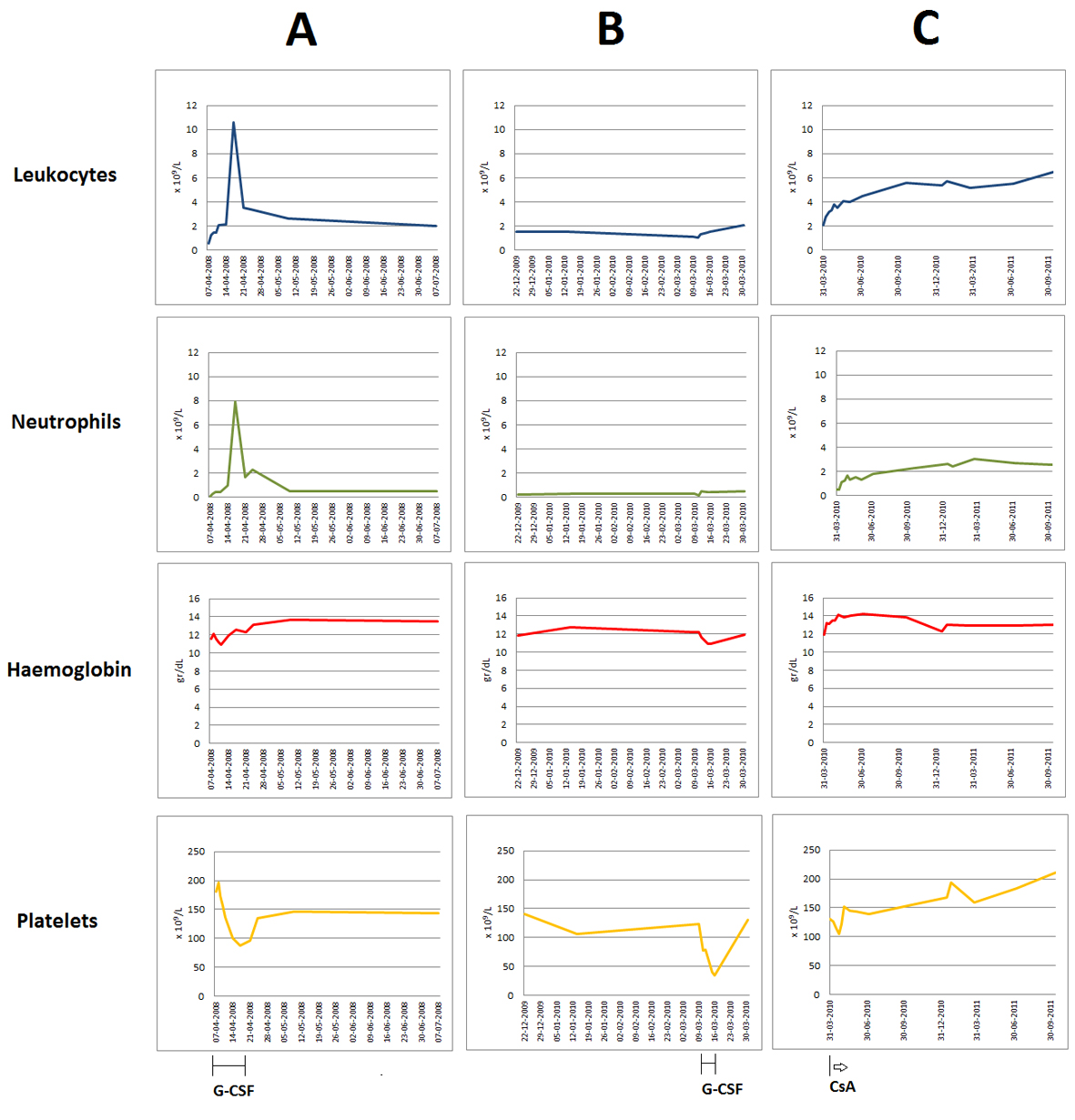 Click for large image | Figure 1. Evolution of the peripheral blood counts (leukocytes, neutrophils, haemoglobin and platelets) over time. Graphics document the chronic severe neutropenia, mild anaemia and mild thrombocytopenia observed in this patient, the two episodes of thrombocytopenia associated with G-CSF administration for undercurrent infections (A, B), as well as the neutrophil recovering after starting on oral CsA, with normalization of all blood counts after long term therapy (C). |
Investigations
Blood counts revealed severe leukopenia and neutropenia (leucocytes 1.5 × 109/L; neutrophils 0.2 × 109/L; lymphocytes 1.4 × 109/L), as well as mild anaemia (haemoglobin 118 g/L) and thrombocytopenia (platelet count 140 × 109/L). The bone marrow aspirate and biopsy showed a normal myeloid/erythroid ratio and a normal granulocytic maturation, without morphological evidence for lymphocytic infiltration. X-rays of the hands and feet revealed subarticular bone erosions, radial aspect of the proximal interphalangeal, metacarpal and metatarsophalangeal joints and soft tissue swelling. The abdominal echography confirmed the splenomegaly (longitudinal and antero-posterior splenic axes of 18 and 13 cm, respectively).
The rheumatoid factor was of 111 UI/mL (normal < 20 UI/mL), the anti-cyclic citrullinated protein antibodies were of > 2,500 UI/mL (normal < 20 UI/mL), and the erythrocyte sedimentation rate was of 35 mm at the end of first hour. Serum IgG was slightly increased (1,690 mg/dL; normal range: 793 - 1,590 mg/dL) and a small monoclonal band (IgG, k) was detected. Biochemical parameters, including creatinine serum levels were normal. Anti-neutrophil IgG antibodies were documented on the neutrophil membrane by flow cytometry.
Peripheral blood immunophenotyping revealed that 93% of lymphocytes were T-cells, from which 74% were TCR-αβ+ (CD4+: 36%, CD8+: 33%) and 26% were TCR-γδ+ CD4- CD8-/+dim T-cells. T-LGL, defined as CD26-, CD27- and CD28- T-cells expressing CTL associated markers, accounted for 66% of CD8+ TCR-αβ+ and for 99% of the TCR-γδ+ T-cell compartments, respectively. TCR-αβ+ CD8+ and TCRγδ+ T-LGL had similar abnormal patterns of expression of CD2, CD5, CD7, CD11c, CD45RA and CD57 antigens (Fig. 2). TCR-Vδ repertoire studies confirmed a major Vd1+ LGL expansion (96% of the TCR-γδ+ T-cells) whereas those from TCR- Vδ showed a dilution pattern, indicating an expansion of one or more TCR-Vδ families that are not detected with the panel of monoclonal antibodies used [2]. Molecular studies using the five EuroClonality (BIOMED-2) standardized PCR-based TCR multiplex tubes [13], provided evidence for a monoclonal TCRG gene rearrangement (Fig. 3A) and revealed a polyclonal TCRB gene rearrangement pattern (Fig. 3B).
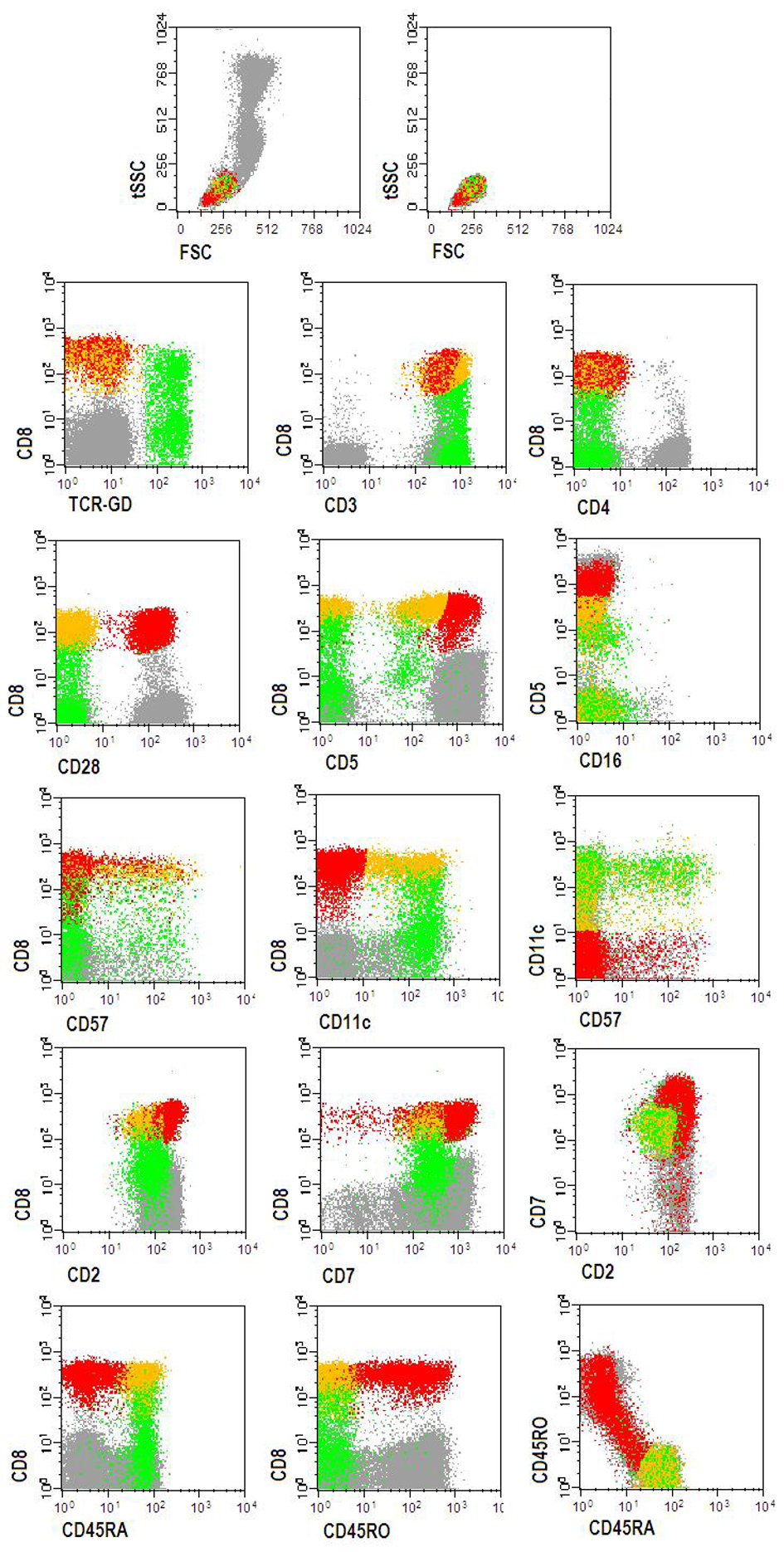 Click for large image | Figure 2. Flow cytometry dot plots showing the abnormal TCRαβ+CD8+strong (yellow dots) and TCRγδ++CD8-/+dim (green dots) T-LGL clones, detected in the peripheral blood. Red dots represent normal CD3+TCRαβ+CD8+strong T-cells. Other peripheral blood lymphocytes are represented by gray dots. Phenotypically abnormal CD3+TCRαβ+CD8+strong (yellow dots) and CD3+TCRγδ+(Vγ+)CD8-/+dim (green dots) T-LGLL cells had a similar immunophenotype for the majority of the markers: CD2+dim, CD3+, CD5-/+dim, CD7+dim, CD11c+,, CD16-, CD28-, CD45RA+, CD45RO-, CD57-/+dim. Other markers (data not shown): CD11a+bright, CD56-, CD94-, HLA-DR-/+dim. |
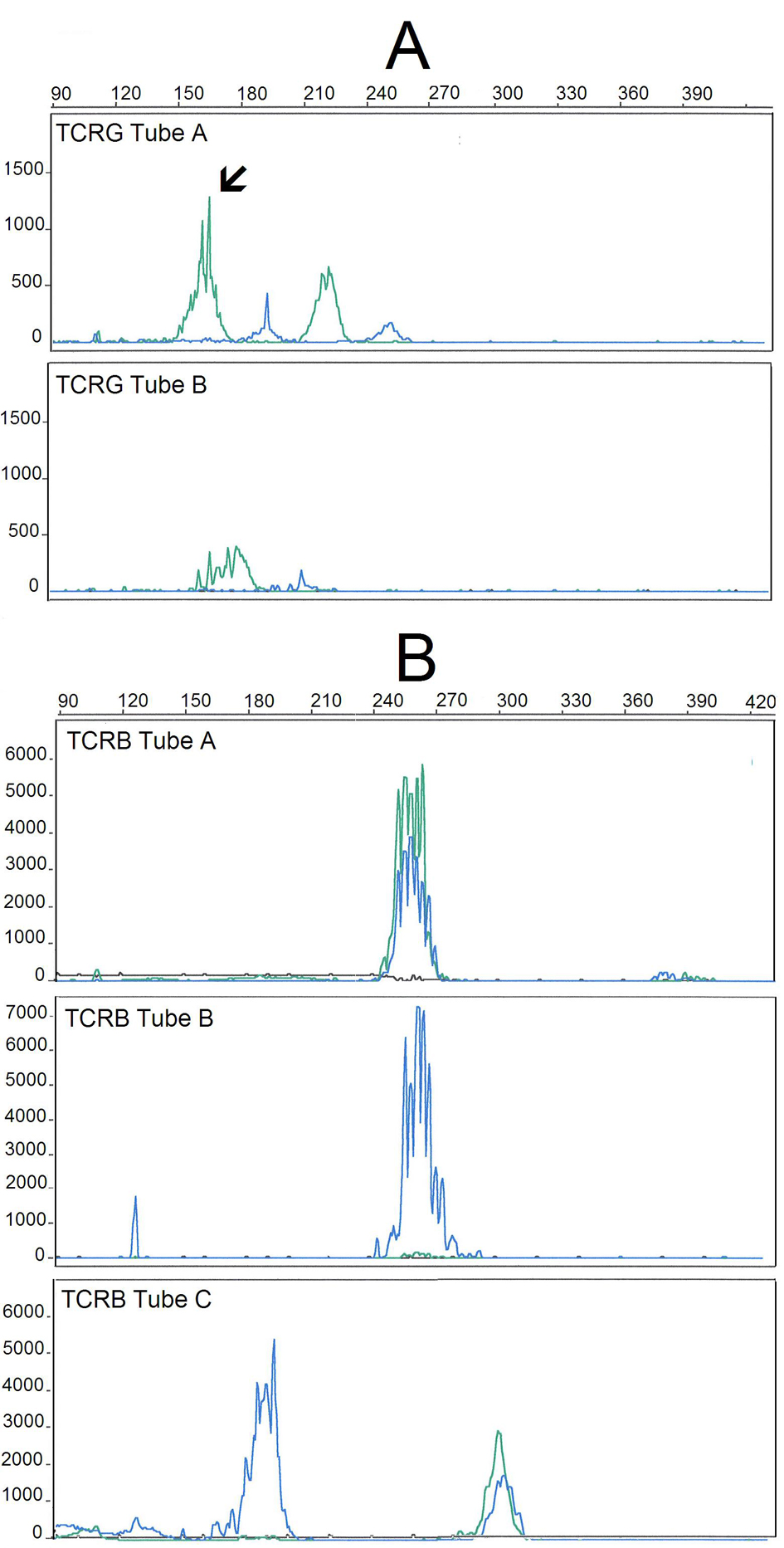 Click for large image | Figure 3. GeneScan fragment analysis of PCR products from TCR gene rearrangements of the DNA extracted from the peripheral blood of the patient, using the five BIOMED-2/EuroClonality multiplex tubes for TCRG (A) and TCRB (B) genes. TCRG and TCRB multiplex tubes are designed with different Vγ/Jγ and Vb/Jb primers, respectively, allowing for the amplification PCR products with different sizes: Vγ/Jγ (240 - 285 nt) and Vb/Jb (285 - 325 nt). Shown are the results obtained in TCRG-tubes A and B (A) and TCRB-tubes A, B and C (B). The analysis of the TCRG gene rearrangements reveals a clonal peak in a polyclonal background (TCRG-tube A, with the arrow indicating the clonal peak) whereas analysis of the TCRB gene rearrangements show a polyclonal pattern, with a typical Gaussian. |
Differential diagnosis
Felty’s syndrome and T-LGL-LPD (including T-LGLL) are not mutually exclusive entities, as the distinction depends mainly on the clinical presentation and on the exhaustiveness of the laboratory investigation rather than being conceptual [6]. In fact, by definition, Felty’s syndrome consists of the triad of AR, splenomegaly, leukopenia with neutropenia and variable lymphocyte counts, whereas T-LGL-LPD, which may also occur in patients with RA, usually manifests as lymphocytosis, with or without splenomegaly and/or neutropenia [6]. Moreover, patients with Felty’s syndrome frequently show clonal T-LGL proliferations, suggesting that these are overlapping entities, and that the distinct clinical manifestations could probably be explained by differences in the homing and functional properties of the clonally expanded T-LGL and in clone size [6].
Treatment
Shortly after being admitted to our consultation, when treatment options were still being discussed, the patient had a skin infection on the face and started on ciprofloxacin and G-CSF (300 µg /day). Five days after, the platelet counts decreased from 123 to 41 × 109/L, without evidence for neutrophil recovering (Fig. 1B). Thus, G-CSF was stopped and the patient was medicated with oral CsA (200 mg twice a day).
Outcome and follow-up
Treatment with CsA resulted in a progressive increase in neutrophil counts and after one month the patient had already 1.1 × 109 neutrophils/L (Fig. 1C). The secondary effects included muscle cramps, hypertension, renal insufficiency and gingival hypertrophy. Muscle cramps were easily controlled with oral magnesium. Mild hypertension (systolic: 140 - 160 mmHg; diastolic: 90 - 100 mmHg) and increased creatinine serum levels (maximum of 2.1 mg/dL) were noted for the first time nearly one month after starting CsA; by adjusting the CsA dosage and adding a long-acting calcium-channel blocker (amlodipine, 5 mg/day), we could control hypertension and prevent further deterioration of the renal function. However, the patient developed mild peripheral oedema with normal cardiac function and progressive gingival hypertrophy, the later being difficult to manage. Besides of the cosmetic effect, it affected the oral hygiene, leading to gingival bleeding and tooth caries and requiring periodically partial gingivectomy. After suspending amlodipine, by July 2012, the peripheral oedema disappeared and the gingival hypertrophy reduced markedly. On the last evaluation, by October 2012, the patient persisted on CsA (125 mg twice a day) and prednisone (5 mg/day), and had normal blood counts, controlled joint complains and absence of infectious episodes.
| Discussion | ▴Top |
We reported on a case of a αβ+/γδ+ T-LGL-LPD manifesting as Felty’s syndrome in a patient with longstanding RA and severe neutropenia, which deserves discussion concerning the diagnosis, the mechanisms underlying the disease manifestations and the therapeutic decisions.
Biological bases for Felty’s syndrome and T-LGL lymphoproliferative disorders
This case fulfils the criteria both for the diagnosis of a Felty’s syndrome and a TCR-αβ+/TCR-γδ+ T-LGL-LPD with restricted TCR-V Vb and TCR- Vδ repertoires and similar aberrant immunophenotyping features. The fact that only monoclonal TCRG genes rearrangements were documented in the case reported herein, could be explained by limitations of the method in distinguishing polyclonal, oligoclonal and monoclonal T cell proliferations in specific conditions, such as low representation of the expanded T-cell clones, large polyclonal background and coexistence of clonally expanded TCR-αβ+ and TCR-γδ+ T cells [14]. The possibility of the TCR-αβ+ and the TCR-γδ+ T-LGL expansions being driven by a common antigen can be hypothesised. In fact, it is now accepted that T-LGL proliferations are triggered and sustained by antigen driven CTL expansions that develop non-randomly in the context of an immune response [5, 15]. This hypothesis could explain why in patients with T-LGL-LPD, similar genetic sequences are shared by multiple dominant and co-dominant T-cell clones, as defined by the Vb-chain complementarity-determining region 3 (CDR3), the antigen-specific portion of the TCR [15].
Possible mechanism for the neutropenia
Neutropenia is usually the most significant problem both in Felty’s syndrome and T-LGLL patients and in generally it cannot be explained by tissue infiltration by the LGL [1, 6, 9]. Possible pathogenic mechanisms include autoantibody or immune complex mediated peripheral destruction of mature neutrophils, induction of Fas/FasL mediated neutrophil apoptosis, autoimmune destruction of the myeloid precursor cells and deregulation of the granulocytic cell maturation [10], probably related to the production of proinflammatory cytokines [16, 17]. In the case reported herein, positive anti-neutrophil autoantibodies and a normal bone marrow, argue for an autoimmune mechanism.
Treatment options Treatment options for neutropenia associated with T-LGL-LPD (including T-LGLL) should be discussed in the light of the best biological and clinical evidence, taking into account the mechanism of action, the efficacy and the side effects, as well as the costs. When this patient was referred to our hospital, the major difficulty was to select the optimal therapy, as he was chronically medicated with corticosteroids, and has been previously treated with immunosuppressive agents and G-CSF without success.
G-CSF effectiveness and drug induced thrombocytopenia
Treatment with G-CSF often improves neutropenia in patients with T-LGL-LPD (including T-LGLL) and Felty’s syndrome, being especially useful when used for short periods of time, during intercurrent infections [9, 10, 18]. Unfortunately, this patient did not show a satisfactory response to G-CSF and suffered from a rare side effect, which is thrombocytopenia. Drug induced thrombocytopenia can be a consequence of decreased platelet production or accelerated platelet destruction [19]. Platelet consumption may occur by different mechanisms, including antibodies that recognize platelet glycoproteins in the presence of the sensitizing drug or of its metabolites, immune complexes or true autoimmune phenomena [20]. Slight thrombocytopenia is commonly observed during G-CSF mobilization of haematopoietic stem cells and has been attributed to the leukapheresis procedure and/or to G-CSF therapy [19], a possible mechanism being G-CSF-induced splenomegaly and hypersplenism [21]. However, the low number of reports on G-CSF induced thrombocytopenia contrast with the high number of patients receiving G-CSF, indicating some kind of individual drug sensitivity. Indeed, only a few cases of clinically significant G-CSF induced thrombocytopenia have been published to date [22-25], one of which documented a severe thrombocytopenia in a patient treated with G-CSF, whose subsequent investigations suggested an underlying immune mechanism [22], and another occurred in a patient with Felty’s syndrome [24]. In our patient, the fact that second exposition to G-CSF resulted in a more rapidly and profound decrease in platelet counts would suggest an immune-mediated mechanism, with an anamnestic response.
Cyclosporine a effectiveness and drug induced gingival hypertrophy
The immunosuppressive agents are the mainstay of therapy in T-LGLL, being effective in about 50-60% of patients [9-12]. However, responders usually require indefinite therapy to prevent relapse, as reversion of neutropenia with CsA and MTX occurs despite the persistence of the abnormal clonally expanded T-LGL, suggesting that the main effect is immunosuppressive or immunomodulatory [11, 12]. CsA acts on the expression of the genes coding for the interleukin 2 (IL-2) and the IL-2 receptor, suppressing both the cellular and the antibody-mediated immune responses [25].
Toxicities of long-term CsA therapy should not be neglected. Thus, after achievement of the best response, the dose should be tapered down slowing in order to obtain the lowest effective dose and to control side effects [9-12]. This patient maintained acceptable and stable neutrophil numbers at the CsA dosage of 125 mg twice/day, with minimal lateral effects except for gingival hypertrophy. In concerning this side effect, it should be emphasised that gingival hyperplasia has also been reported in association with calcium channel blockers, especially with dihydropyridines or benzothiazepine derivatives [26], and adding amlodipine in an attempt to control hypertension and renal dysfunction has markedly contributed to worsening of this condition.
Learning points/take home messages
Consider the diagnosis of a T-LGL-LPD (including T-LGLL) in patients with chronic neutropenia, especially if associated with RA, and keep in mind that it may be manifest as Felty’s syndrome.
Remember that clonal T-LGL expansions may occur without lymphocytosis, and their identification requires laboratory expertise concerning morphology, immunophenotyping and molecular studies.
Ponder CsA for long-term treatment of severe and/or symptomatic neutropenia associated with clonal T-LGL proliferations, adapting CsA dosage to each patient in order to obtain the desired therapeutic benefit with minimal toxicity and avoiding other medications with overlapping side effects, such as calcium channel blockers because they may also cause gingival hypertrophy.
Consider G-CSF to control neutropenia for short periods of time, providing that the patient is responsive, and investigate the possibility of drug induced thrombocytopenia when it develops de novo (or worsens) in patients treated with G-CSF.
Acknowledgments
The authors thank to Professor Carlos Vasconcelos, from the Unit of Clinical Immunology, Hospital de Santo Antonio, Centro Hospitalar do Porto, for his contribution concerning patient care and therapeutic decisions. The authors also thank to other medical doctors and laboratory technicians from the Laboratory of Cytometry, especially to Ana Helena Santos and Joao Rodrigues for technical support concerning flow cytometry and molecular genetics studies.
| References | ▴Top |
- Sokol L, Loughran TP, Jr. Large granular lymphocyte leukemia. Oncologist. 2006;11(3):263-273.
doi pubmed - Lima M, Almeida J, Santos AH, dos Anjos Teixeira M, Alguero MC, Queiros ML, Balanzategui A, et al. Immunophenotypic analysis of the TCR-Vbeta repertoire in 98 persistent expansions of CD3(+)/TCR-alphabeta(+) large granular lymphocytes: utility in assessing clonality and insights into the pathogenesis of the disease. Am J Pathol. 2001;159(5):1861-1868.
doi - Sandberg Y, Almeida J, Gonzalez M, Lima M, Barcena P, Szczepanski T, van Gastel-Mol EJ, et al. TCRgammadelta+ large granular lymphocyte leukemias reflect the spectrum of normal antigen-selected TCRgammadelta+ T-cells. Leukemia. 2006;20(3):505-513.
doi pubmed - Lima M, Almeida J, Dos Anjos Teixeira M, Alguero Md Mdel C, Santos AH, Balanzategui A, Queiros ML, et al. TCRalphabeta+/CD4+ large granular lymphocytosis: a new clonal T-cell lymphoproliferative disorder. Am J Pathol. 2003;163(2):763-771.
doi - Garrido P, Ruiz-Cabello F, Barcena P, Sandberg Y, Canton J, Lima M, Balanzategui A, et al. Monoclonal TCR-Vbeta13.1+/CD4+/NKa+/CD8-/+dim T-LGL lymphocytosis: evidence for an antigen-driven chronic T-cell stimulation origin. Blood. 2007;109(11):4890-4898.
doi pubmed - Liu X, Loughran TP, Jr. The spectrum of large granular lymphocyte leukemia and Felty's syndrome. Curr Opin Hematol. 2011;18(4):254-259.
doi pubmed - Rodriguez-Caballero A, Garcia-Montero AC, Barcena P, Almeida J, Ruiz-Cabello F, Tabernero MD, Garrido P, et al. Expanded cells in monoclonal TCR-alphabeta+/CD4+/NKa+/CD8-/+dim T-LGL lymphocytosis recognize hCMV antigens. Blood. 2008;112(12):4609-4616.
doi pubmed - Epling-Burnette PK, Loughran TP, Jr. Survival signals in leukemic large granular lymphocytes. Semin Hematol. 2003;40(3):213-220.
doi - Lamy T, Loughran TP, Jr. How I treat LGL leukemia. Blood. 2011;117(10):2764-2774.
doi pubmed - Pontikoglou C, Kalpadakis C, Papadaki HA. Pathophysiologic mechanisms and management of neutropenia associated with large granular lymphocytic leukemia. Expert Rev Hematol. 2011;4(3):317-328.
doi pubmed - Sood R, Stewart CC, Aplan PD, Murai H, Ward P, Barcos M, Baer MR. Neutropenia associated with T-cell large granular lymphocyte leukemia: long-term response to cyclosporine therapy despite persistence of abnormal cells. Blood. 1998;91(9):3372-3378.
pubmed - Loughran TP, Jr., Kidd PG, Starkebaum G. Treatment of large granular lymphocyte leukemia with oral low-dose methotrexate. Blood. 1994;84(7):2164-2170.
pubmed - van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257-2317.
doi pubmed - Langerak AW, Groenen PJ, Bruggemann M, Beldjord K, Bellan C, Bonello L, Boone E, et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012;26(10):2159-2171.
doi pubmed - Wlodarski MW, O'Keefe C, Howe EC, Risitano AM, Rodriguez A, Warshawsky I, Loughran TP, Jr., et al. Pathologic clonal cytotoxic T-cell responses: nonrandom nature of the T-cell-receptor restriction in large granular lymphocyte leukemia. Blood. 2005;106(8):2769-2780.
doi pubmed - Garrido P, Almeida J, Romero JM, Canton J, Sandberg Y, Barcena P, Lima M, et al. Evaluation of functional single nucleotide polymorphisms of different genes coding for the immunoregulatory molecules in patients with monoclonal large granular lymphocyte lymphocytosis. Hum Immunol. 2008;69(2):101-107.
doi pubmed - Kothapalli R, Nyland SB, Kusmartseva I, Bailey RD, McKeown TM, Loughran TP, Jr. Constitutive production of proinflammatory cytokines RANTES, MIP-1beta and IL-18 characterizes LGL leukemia. Int J Oncol. 2005;26(2):529-535.
pubmed - Newman KA, Akhtari M. Management of autoimmune neutropenia in Felty's syndrome and systemic lupus erythematosus. Autoimmun Rev. 2011;10(7):432-437.
doi pubmed - Anderlini P, Przepiorka D, Champlin R, Korbling M. Biologic and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood. 1996;88(8):2819-2825.
pubmed - Visentin GP, Liu CY. Drug-induced thrombocytopenia. Hematol Oncol Clin North Am. 2007;21(4):685-696, vi.
doi pubmed - Picardi M, De Rosa G, Selleri C, Scarpato N, Soscia E, Martinelli V, Ciancia R, et al. Spleen enlargement following recombinant human granulocyte colony-stimulating factor administration for peripheral blood stem cell mobilization. Haematologica. 2003;88(7):794-800.
pubmed - Kovacic JC, Macdonald P, Freund J, Rasko JE, Allan R, Fernandes VB, Ma D, et al. Profound thrombocytopenia related to G-CSF. Am J Hematol. 2007;82(3):229-230.
doi pubmed - Minelli O, Falzetti F, Di Ianni M, Onorato M, Plebani S, Silvani C, Tabilio A. G-CSF-induced thrombocytopenia in a healthy donor. Bone Marrow Transplant. 2009;43(3):263-264.
doi pubmed - Wun T. The Felty syndrome and G-CSF-associated thrombocytopenia and severe anemia. Ann Intern Med. 1993;118(4):318-319.
doi pubmed - Calder VL, Bellamy AS, Owen S, Lewis C, Rudge P, Davison AN, Feldmann M. Effects of cyclosporin A on expression of IL-2 and IL-2 receptors in normal and multiple sclerosis patients. Clin Exp Immunol. 1987;70(3):570-577.
pubmed - Kaur G, Verhamme KM, Dieleman JP, Vanrolleghem A, van Soest EM, Stricker BH, Sturkenboom MC. Association between calcium channel blockers and gingival hyperplasia. J Clin Periodontol. 2010;37(7):625-630.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


