| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 1, Number 1, August 2010, pages 1-3
Unusually Severe Bullous Skin Reaction to Sorafenib: A Case Report
Benjamin Hagopiana, c, Clifford D Packera, b
aCase Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106, USA
bLouis Stokes Cleveland VA Medical Center, 10701 East Blvd, Cleveland, OH 44106, USA
cCorresponding author: Benjamin Hagopian
Manuscript accepted for publication July 1, 2010
Short title: Bullous Skin Reaction to Sorafenib
doi: https://doi.org/10.4021/jmc112e
| Abstract | ▴Top |
While there are multiple reports on sorafenib-induced hand foot skin reaction, this case report details a patient presenting with an unusually severe and painful skin reaction. As the armamentarium of anti-neoplastic kinase inhibitors continues to increase, clinicians must be aware of the array of skin reactions these drugs can induce. A 66 years old Caucasian male with metastatic renal cell carcinoma presented with flu-like symptoms, rash, and painful bullous lesions of his fingertips. After empiric coverage with antibiotics and an infectious workup that turned up negative, it was determined that the patient’s presentation was due to a severe and painful hand skin reaction to sorafenib. While most patients taking sorafenib will experience a cutaneous side effect, the hand foot skin reaction can be extremely debilitating and occurs frequently. Oncologists and dermatologists must be informed about the array of adverse effects of kinase inhibitors, as treatment adherence and outcomes are at stake. In this case report, the bullous skin reaction, debilitating hand pain, and absence of foot involvement were the interesting features.
Keywords: Sorafenib; Hand foot skin reaction
| Introduction | ▴Top |
Sorafenib is a multikinase inhibitor used to treat advanced renal cell, hepatocellular, and thyroid cancers [1]. It inhibits a variety of kinases, enzymes that are involved in the transfer of phosphate groups from ATP to a substrate molecule; the groundbreaking drug Gleevec (imatinib) is a well known kinase inhibitor and is similar to sorafenib [2]. Specifically, sorafenib inhibits both intracellular Raf kinases (such as BRAF) as well as cell surface kinase receptors (such as VEGFR-2) [1].
While sorafenib has a plethora of side effects such as hypertension and diarrhea, the cutaneous side effects of the drug are among the most common and devastating. Documented dermatologic complications of sorafenib include facial erythema, subungual splinter hemorrhages, dry skin, pruritis, alopecia, scalp dysesthesia, and the hand-foot skin reaction (HFSR), often characterized by hyperkeratosis, involving the palms and soles. In a small study of 43 patients on sorafenib, more than 90% developed cutaneous symptoms [3].
| Case Report | ▴Top |
A 66 years old Caucasian male with a history of renal cell carcinoma with bilateral lung metastases presented to our urgent care center with a chief complaint of fever, fatigue, malaise, weakness, anorexia, one episode of emesis, and discoloration and pain of his finger tips for two days. At admission, the patient’s chemotherapy regimen consisted of sorafenib, which he had been taking for approximately six weeks, after being switched from sunitinib because of pancreatitis. The patient had been seen two and a half weeks earlier for similar flu-like symptoms and fingertip pain; at that time, his sorafenib dose was reduced.
Past medical history was significant for diabetes mellitus, hypertension, dyslipidemia, and stage 3 chronic kidney disease. He had undergone a right nephrectomy two years earlier for his renal cell carcinoma. He had quit smoking approximately 12 years before, and denied alcohol and illicit drug use. His home medications included sorafenib at 200 mg twice per day, atenolol, fosinopril, ciprofloxacin, HCTZ, nifedipine, simvastatin, baby aspirin, glipizide, and insulin.
On physical exam, the patient’s vitals were temperature 36.3°C, pulse rate 92/min, respiration 20/min, and blood pressure 152/68 mm Hg. He was alert and oriented x 3. His eyelids were swollen and crusted. His skin showed mottled lesions over the finger tips, with swollen and tender finger pads. There were also cyanotic nodules over the dorsal aspect of the finger tips as well as acneiform lesions on his upper back. Lungs were clear to auscultation and the heart had a regular rate and rhythm without murmurs or rubs. The abdomen was soft and non-tender, with no masses or organomegaly. Pertinent lab values included white blood cell count of 8.75 x 103/mm3, hematocrit 30.9% (slightly below baseline), sodium 126 mmol/L, albumin 2.5g/L, and creatinine 2 mg/L. Chest x-ray was normal.
During his hospital stay, the patient’s finger bullae began to enlarge and coalesce (Fig. 1). The bullae progressed from violaceous to pale blue, with severe pain (the patient was unable to pick up utensils to feed himself). Some of the lesions spontaneously erupted and drained. Finally, the lesions became flattened and dark-blue to black in color, with significant improvement in the pain.
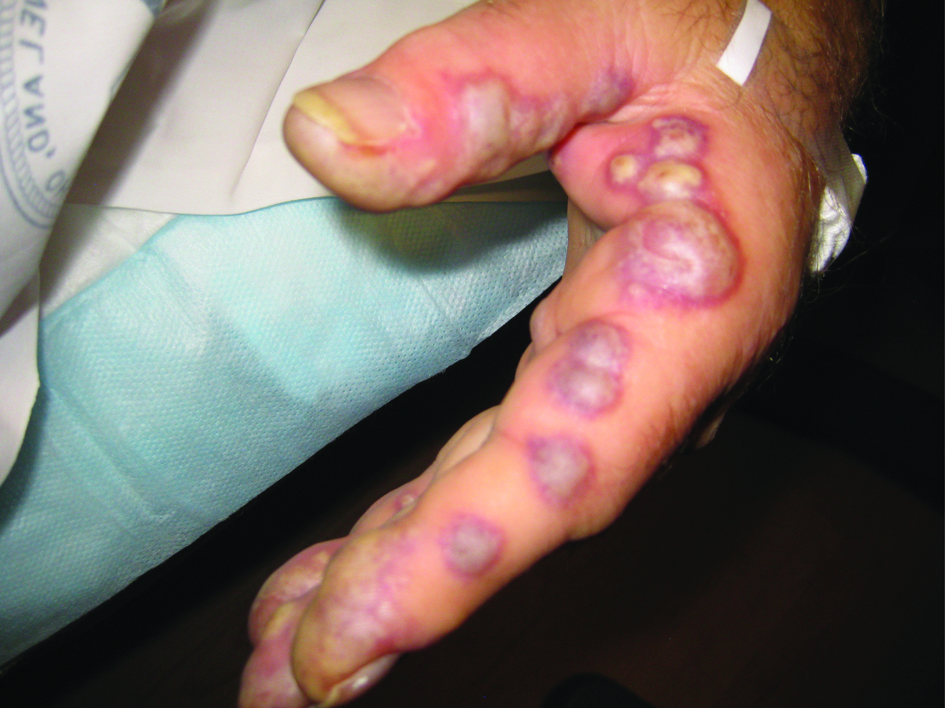 Click for large image | Figure 1. Photo of bullous lesions on patient’s left hand. This picture was taken when both hands had many bullous violaceous lesions. Note the discoloration of the finger tips as well as the bullae along the lateral side of the left index finger. The patient was in such severe pain at this point in his hospital course that he held his hands out in front of him, for fear of the lesions coming into contact with any surface. Later in the hospital course, the lesions erupted, drained, darkened, and became less painful. |
Upon admission, the differential diagnosis consisted of infectious process (sepsis, endocarditis) versus an adverse reaction to sorafenib. The patient was empirically treated with pipericillin/tazobactam until blood cultures came back negative. He was given hydromorphone for pain control, and the sorafenib was discontinued. His acneiform back lesions were biopsied, returning as neutrophilic dermatosis, which supported sorafenib as a cause of the rash (Fig. 2). After the lesions began to improve, he was discharged with hydromorphone for pain and an appointment with his oncologist to discuss other options for treating his cancer.
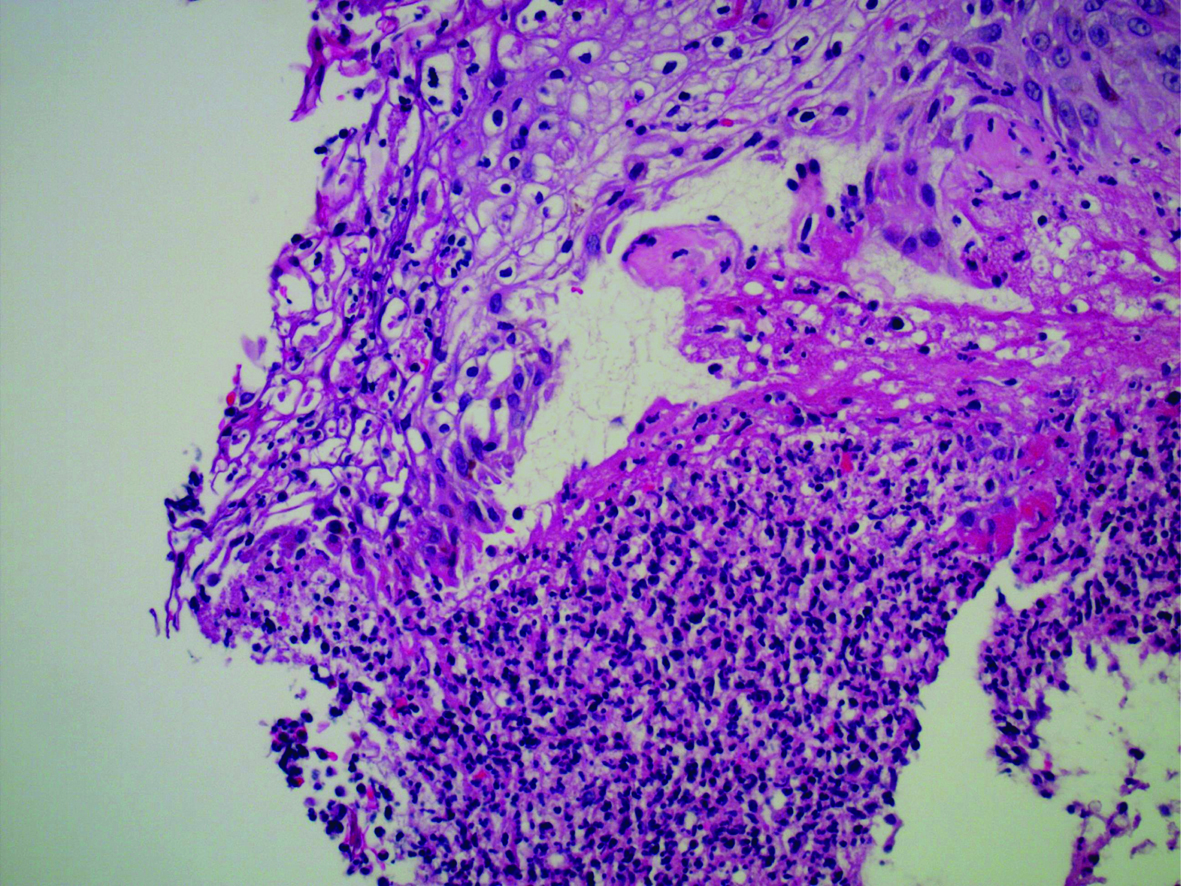 Click for large image | Figure 2. Photomicrograph of acneiform back lesions. This photomicrograph displays the results of the patient’s acneiform back lesion biopsy. Note the ulceration along the top left of the image as well as the neutrophilic infiltration at the bottom. Stained with hematoxylin and eosin, 400x magnification. |
| Discussion | ▴Top |
HFSR is a relatively common dermatologic side effect associated with the use of sorafenib. In a meta-analysis, the overall incidence of HFSR for patients taking sorafenib for metastatic tumors was 34% [4]. The severity of HFSR has been delineated by the National Cancer Institute into three grades. Grade one HFSR is characterized by “minimal changes or dermatitis without pain”, grade two is described as “skin changes or pain, not interfering with function”, and grade three is “ulcerative dermatitis or skin changes with pain interfering with function [3].” Treatment consists of topical therapy such as creams, dose reduction, or treatment discontinuation, usually with resolution of symptoms [1, 3].
The pathophysiology of chemotherapy-induced HFSR is not well understood. However, various hypotheses have been proposed to explain the reaction. These include increased drug delivery to areas with a high concentration of eccrine sweat glands, a dose-dependent direct toxic effect on the skin, and blockade of VEGF and PDGF receptors [3, 5].
The likelihood that sorafenib caused this patient’s skin lesions is high. The timing of the skin reaction, improvement with cessation of sorafenib, suggestive skin biopsy findings, and absence of other likely causes all support the diagnosis. The Naranjo ADR probability scale score of 7 strongly suggests that sorafenib was the probable cause [6].
Our patient had an unusually severe reaction to sorafenib compared to most adverse reactions described in the literature. First, his cutaneous reaction to sorafenib was a grade 3 reaction; a meta-analysis has estimated the incidence of grade 3 HFSR at 9% among the studied patients with sorafenib-induced HFSR [4]. Also, he had bullous involvement of his hands; in the study of 43 patients on sorafenib, only one patient developed bullous lesions [3]. Furthermore, it was interesting to note that, given the severity of the patient’s reaction, the HFSR was limited to the hands, with no foot involvement.
In summary, our patient had a severe hand skin reaction to the novel chemotherapeutic agent sorafenib, consisting of extremely painful bullous lesions of the fingers without foot involvement, and an acneiform rash on the upper back. Because a large percentage of patients on sorafenib will experience some degree of HFSR and because the associated symptoms can be devastating, as displayed in this case report, oncologists must educate their patients about HFSR and other dermatologic symptoms before sorafenib treatment is initiated, in order to maintain medication adherence and maximize positive clinical outcomes.
Acknowledgments
Thank you to Dr. Waqas Rahman for his assistance in acquiring patient data. Thank you to Dr. Medhat Hassan for his assistance with the pathology slide.
Competing Interests
The authors declare that they have no competing interests.
Use of Patient Information
The authors assert that they have adhered to their institution’s policy governing use of patient information.
Authors' Contributions
BH obtained the patient history, performed the literature review, and wrote the manuscript. CP obtained the Naranjo ADR score as well as the patient’s pathology and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
| References | ▴Top |
- UpToDate online/Lexi-Comp drug information, article on Sorafenib, [www.utdol.com] accessed 14 February 2010.
- Harvard Magazine, “A Cancer Battlefield Glossary”, Jan-Feb 2007, [http://harvardmagazine.com/2007/01/a-cancer-battlefield-glo.html]. Accessed 14 February 2010.
- Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol 2008;144(7):886-892.
pubmed doi - Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol 2008;47(2):176-186.
pubmed doi - Lai SE, Kuzel T, Lacouture ME. Hand-foot and stump syndrome to sorafenib. J Clin Oncol 2007;25(3):341-343.
pubmed doi - Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30(2):239-245.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


