| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 4-5, May 2024, pages 82-91
Improvements in Gut Microbiome Composition and Clinical Symptoms Following Familial Fecal Microbiota Transplantation in a Nineteen-Year-Old Adolescent With Severe Autism
Sabine Hazana, b, e, Jonathan Haroonc, Sheldon Jordanc, Stephen J. Walkerd
aProgenaBiome, LLC, Ventura, CA, USA
bMicrobiome Research Foundation, Ventura, CA, USA
cThe Regenesis Project, Santa Monica, CA, USA
dWake Forest Institute for Regenerative Medicine, Winston Salem, NC, USA
eCorresponding Author: Sabine Hazan, ProgenaBiome, LLC, Ventura, CA, USA
Manuscript submitted March 28, 2024, accepted April 11, 2024, published online May 2, 2024
Short title: Autism and Fecal Microbiota Transplantation
doi: https://doi.org/10.14740/jmc4209
| Abstract | ▴Top |
This case report describes a novel therapy for patients with severe autism spectrum disorder (ASD) that is worth further investigation. A 19-year-old male adolescent with ASD, who was not responding to standard treatment received fecal microbiota transplant (FMT) using donor material from his typically developing female sibling. The patient’s ASD symptoms were assessed by assessors who were blind to the patient’s past ASD symptomatology. Assessors used the Childhood Autism Rating Scale (CARS), an observation-based rating scale to assess developmental delay in children with autism (range of CARS scores is 15 - 60; a score > 28 is indicative of autism; higher score is positively correlated with degree of severity), at baseline and again at six timepoints post-FMT. The patient experienced marked improvements in microbiome diversity and composition over the year and a half period that followed the FMT procedure. Additionally, the patient who was previously nonverbal said his first two words and experienced a reduction in aggression 1-month post-FMT. To the authors’ knowledge, this is the first report to demonstrate the use of familial FMT in an adolescent patient with ASD. Given that ASD symptom improvements post-FMT tend to occur in younger patients, the authors hypothesize that the use of a familial donor may be an important factor that contributed to the improved outcomes experienced by this older child.
Keywords: Autism spectrum disorder; Fecal microbiota transplantation; Gastrointestinal microbiome; Gut dysbiosis; Bifidobacterium; Proteobacteria; Actinobacteria; Lactobacillus animalis
| Introduction | ▴Top |
Autism spectrum disorder (ASD) is characterized by impaired socialization, altered verbal and non-verbal communication, repetitive behaviors, and restricted interests. Infectious and inflammatory factors appear to disrupt development and therefore potentially remediable factors such as gut dysbiosis have been the subject of some interest. To date, improvements in ASD outcomes post microbiota manipulation have been reported primarily in younger patients, while evidence in older patients is scarce. This, perhaps, reflects a sense of futility in attempts at mitigation for those in the later stages of development [1-7].
In the United States, the prevalence of ASD has risen in recent decades, with current estimates of approximately 1 in 44 children aged 8 years [5]. The increased prevalence of ASD is attributable to multiple factors including a broadening of the diagnostic criteria, increased screening practices, the substitution of an ASD diagnosis in children previously diagnosed with other similar conditions and increased public awareness of ASD symptoms [3, 7]. Specific etiologic factors underlying an ASD diagnosis are often unknown, however, inflammatory factors have come under increased scrutiny in recent years [3, 4, 7, 8].
Alterations in the gut microbiome are associated with several neurological disorders including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [9-15]. In ASD, gut dysbiosis may disrupt the gut-brain axis [16-18]. The presence of gut dysbiosis has engendered the “inflammation hypothesis” as a major underlying mechanism allowing passage of bacteria and metabolites that activate the release of inflammatory cytokines into the bloodstream. The effects of this inflammatory cascade are thought to underlie pathogenesis of ASD by influencing early brain development [16, 18]. This hypothesis is supported by evidence that some children with ASD tend to exhibit increased relative abundances of inflammation-promoting gut microbes such as Clostridium, decreased relative abundances of anti-inflammatory microbes such as Bifidobacterium, and increased plasma levels of proinflammatory interleukins, tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β [16-18]. However, an exact microbiome profile specific to ASD is not yet known, as multiple confounding factors such as age, diet, medication environment, and presence of other comorbidities can influence gut microflora composition and inflammatory state [18].
Modulation of the gut microbiome is emerging as a potential therapeutic option to manage and alleviate symptoms associated with a variety of conditions within and beyond the gastrointestinal system. Fecal microbiota transplant (FMT), a procedure involving infusion of fecal material from a donor into the lower gastrointestinal tract of the recipient, is a particularly powerful method for modifying gut microflora composition because it allows a total exchange of microorganisms [19, 20]. This approach has been used to treat Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and myoclonus-dystonia, with emerging evidence in children with ASD [16, 19, 21-32]. To date, the ASD FMT-trial subjects have predominantly been young; perhaps a reflection of the widely held perception that older children with ASD are less likely to experience benefit. Furthermore, previous studies included healthy donors that were not family members of the ASD subjects [33, 34]. This case report describes the outcomes on the gut microbiome and ASD symptoms in a 19-year-old patient with ASD following familial FMT. To the authors’ knowledge, this is the first report of familial FMT in an adolescent patient with ASD.
| Case Report | ▴Top |
Investigations
After years of unsuccessful treatments with nutraceuticals, such as vitamins C, D, zinc, a diet rich in gut health supplements, and visits to a number of different medical practitioners, this 19-year-old male patient with severe ASD and gastrointestinal symptoms was evaluated for FMT therapy. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration and approved by the Salus Institutional Review Board (IRB, #21064) on April 20, 2021.
Family history
The patient’s mother reported a normal pregnancy with him, and he was delivered vaginally. Family history revealed Alzheimer’s in the maternal grandmother and irritable bowel syndrome in the mother. The child’s father and siblings had no family history of medical issues.
Medical history
The patient received the normal pediatric vaccine regimen. The patient had been exhibiting signs of ASD since the age of 1 year, which included delayed motor milestones, self-injurious stimulatory behaviors, and periods of intense restless behavior and aggression interspersed with periods of relative calmness. Prior medical history noted jaundice at birth which resolved within few days, a remote history of seizures at 3 years old, and duodenitis and gastritis. He also experienced frequent gastrointestinal symptoms: diarrhea, bloating, and constipation.
Symptoms
At the time of evaluation, the patient presented as alert but restless, making frequent vocalizations without functional words, and exhibiting self-stimulatory chest-rubbing movements. He had a recent history of taking vancomycin due to gut dysbiosis, which resulted in a further reduction in his microbiome diversity with no improvements in his aggression or ability to speak. However, he had no history of other courses of antibiotic exposure.
Physical examination, laboratory tests’ results
Complete blood count (CBC) showed white blood cell (WBC) 6.1 × 109/L (normal range: 4.5 to 11.0 × 109/L), hemoglobin 14.6 g/dL (normal range: 14 - 18 g/dL), hematocrit 42.1% (normal range for males: 40-54%), platelets 204 × 109/L (normal range: 150 - 400 × 109/L); other laboratory tests results included: blood urea nitrogen (BUN) 13 mg/dL (normal range: 6 - 20 mg/dL), creatinine 0.75 mg/dL (normal range for males: 0.7 - 1.3 mg/dL), sodium 139 mEq/L (normal range: 135 - 145 mEq/L), potassium 4.0 mEq/L (normal range: 3.5 - 5.2 mEq/L), calcium 10 mg/dL (normal range: 8.5 - 10.3 mg/dL), aspartate aminotransferase (AST) 18 U/L (normal range: 8 - 33 U/L), and alanine aminotransferase (ALT) 20 U/L (normal range: 4 - 36 U/L).
Diagnosis and treatment
The patient’s caregiver provided written informed consent (Salus IRB #21064) and the FMT protocol, described earlier [35], was approved by the Food and Drug Administration (FDA) as an investigational new drug (NCT04878718).
FMT procedure and gut microbiome analysis
Vancomycin was given (500 mg orally, three times a day) 10 days prior to the procedure. A deep colonic wash was administered prior to colonoscopy. The patient underwent a single 300 mL infusion of stool sample (transplant material) directly into the cecum via colonoscopy. The stool was donated by his 15-year-old typically developing sister, who did not have any medical diagnoses of chronic diseases, did not receive antibiotics for a long duration or rarely received antibiotics, and she was on no medications. Fecal samples were collected at baseline from the patient (fecal samples archived prior to vancomycin exposure) and the donor, and from the patient at post-FMT months 2, 6, 8, 11, and 15. Microbiome composition analysis was performed on fecal samples using metagenomic next-generation sequencing, during which DNA samples were extracted and normalized for library downstream fabrication using shotgun methodology, as reported previously [35-37].
Follow-up and outcomes
Assessment of behavioral symptoms
The patient’s ASD symptoms were assessed by assessors who were blind to the patient’s past ASD symptomatology. Assessors used the Childhood Autism Rating Scale (CARS) [38], an observation-based rating scale to assess developmental delay in children with autism (range of CARS scores is 15 - 60; a score > 28 is indicative of autism; higher score is positively correlated with degree of severity), at baseline and again at six timepoints post-FMT.
Improvements in gut microbiome composition
Table 1 displays Shannon index of bacterial diversity at species level for the patient and donor at baseline, and for the patient at post-FMT months 2, 6, 8, 11, and 15. The patient’s microbiome diversity progressively increased during the post-FMT follow-up period, nearly matching the donor’s Shannon index by 6 months post-FMT. This improvement was maintained to at least 15 months post-FMT.
 Click to view | Table 1. Shannon Index of Bacterial Diversity |
Figure 1a displays the donor’s gut microbiome profile at baseline, as well as the patient’s gut microbiome profile at baseline pre-FMT, and at post-FMT months 2, 6, 8, 11, and 15. Species Lactobacillus animalis, absent from the donor, was present in the patient at baseline (relative abundance of 37.14%), disappearing by 6 months post-FMT (relative abundance of 0.00%) (Fig. 1b). Figure 2a specifically displays the presence of phylum profile in the donor as well as the patient at baseline and post-FMT. From baseline to 15 months post-FMT, the patient experienced a decrease in relative abundance of phylum Proteobacteria from 26.42% to 0.99% (Fig. 2b). The patient also experienced an increase in relative abundance of phylum Actinobacteria from 0.00% to 3.03% from baseline to 15 months post-FMT (Fig. 2c), largely due to an increase in the relative abundance of genus Bifidobacterium from 0.00% to 1.56% (Fig. 3).
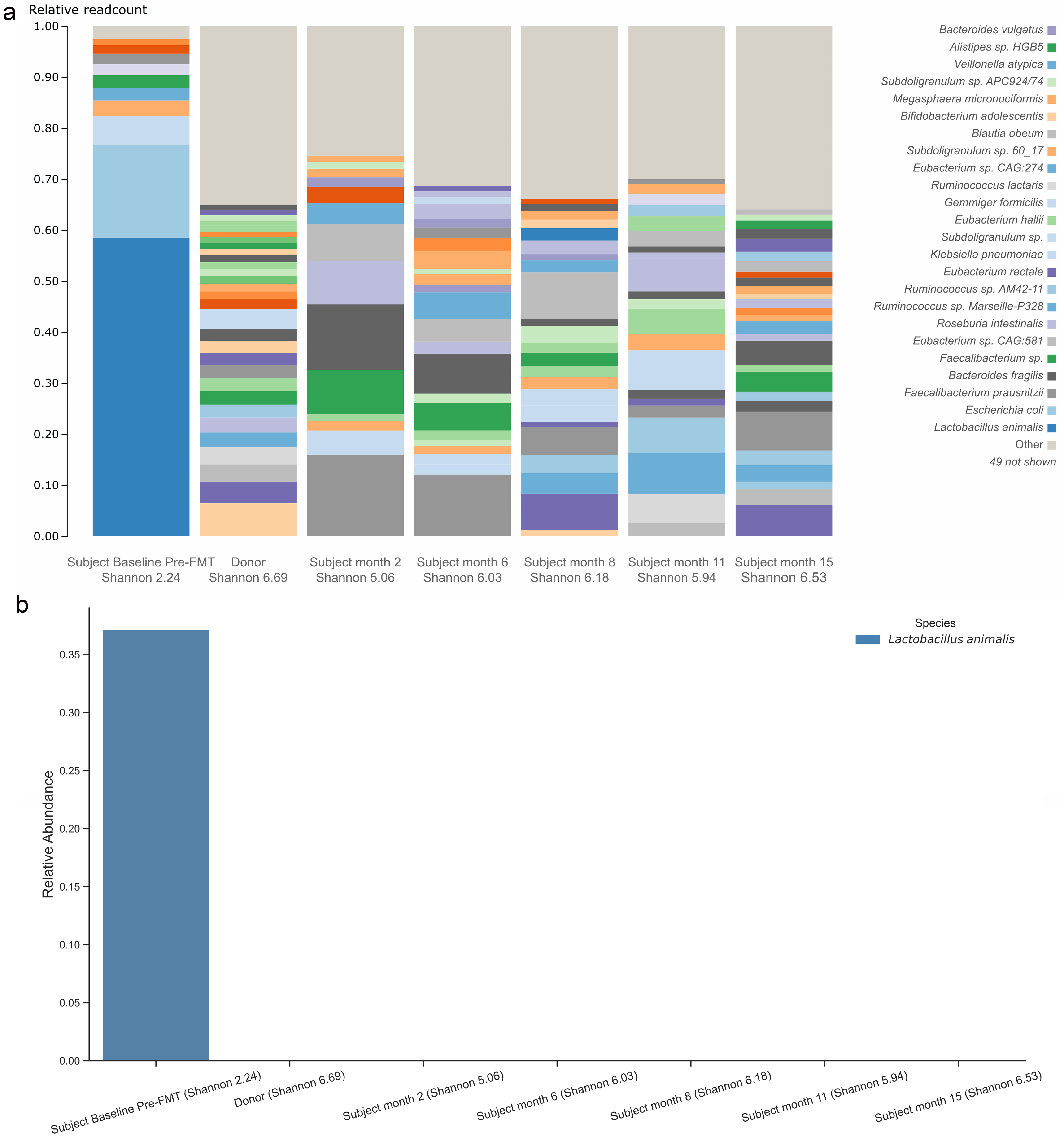 Click for large image | Figure 1. Gut microbiome profile in species level for the patient and donor (a). Species Lactobacillus animalis relative abundance in donor and subject (b). FMT: fecal microbiota transplant. |
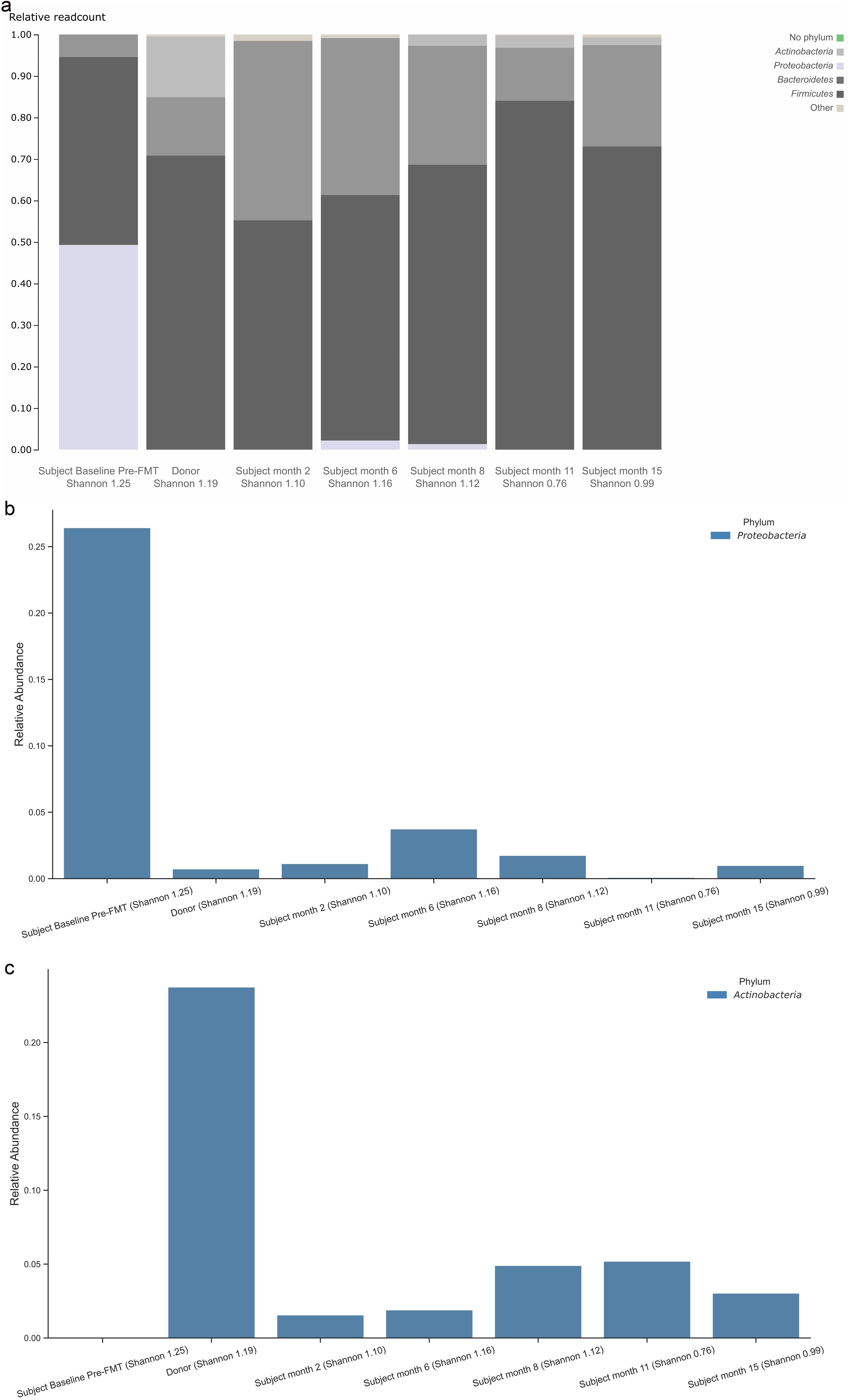 Click for large image | Figure 2. Gut microbiome profile in phylum level for the patient and donor (a). Relative abundance in phylum Proteobacteria (b). Relative abundance in phylum Actinobacteria (c). FMT: fecal microbiota transplant. |
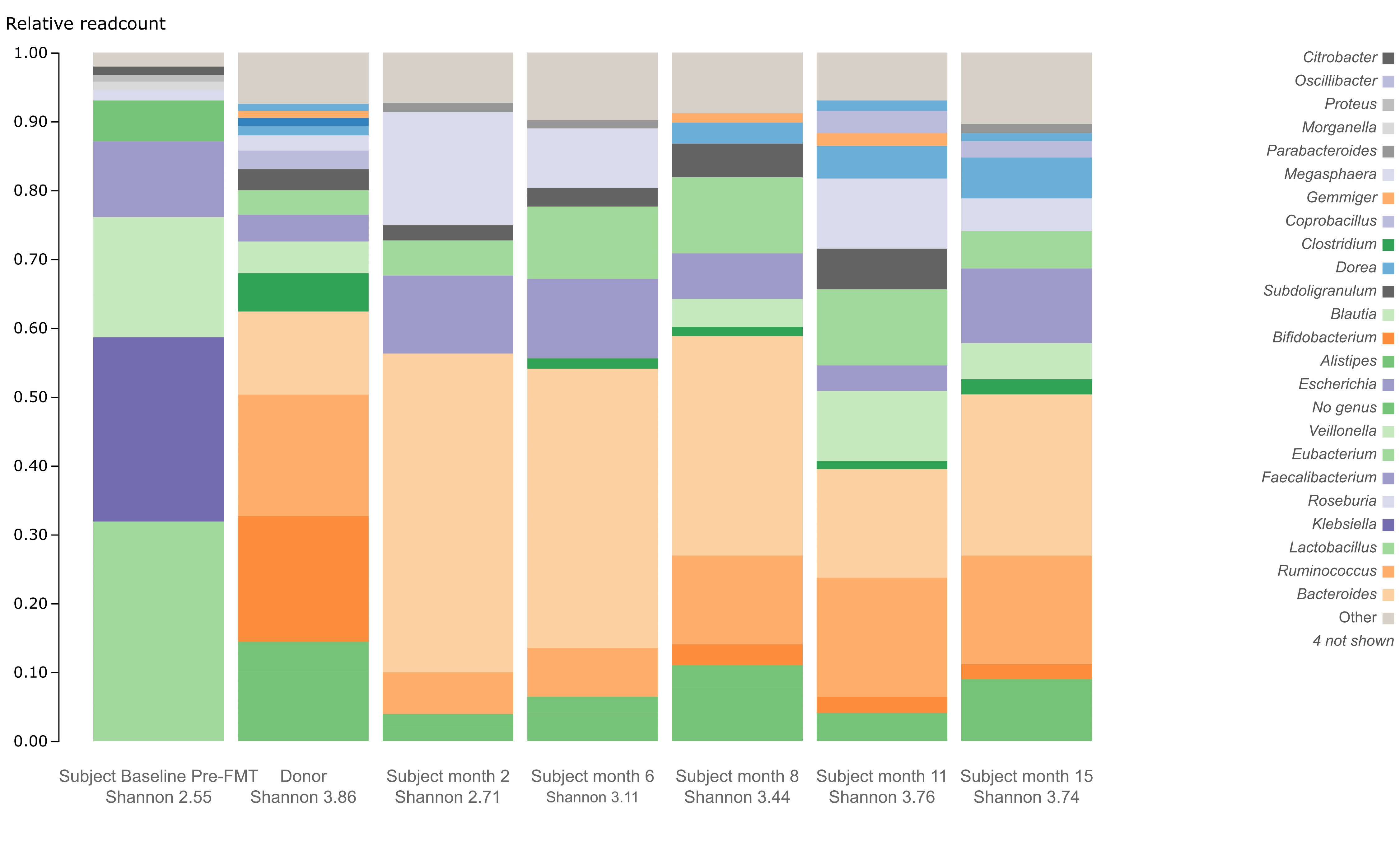 Click for large image | Figure 3. Gut microbiome profile in genus level for the subject and donor. FMT: fecal microbiota transplant. |
Improvements in behavioral and gastrointestinal symptoms
From baseline to month 16 post-FMT, the subject’s CARS scores decreased from 51 to 33.5 (Fig. 4). The Autism Treatment Evaluation Checklist (ATEC) of speech increased from 7 to 16.3, and ATEC of sensory awareness increased from 9 to 24 (Fig. 5). The subject also experienced decrease in aggression, improvement in sleep patterns and said his first two words (“mama” and “baba”) at 1-month post-FMT.
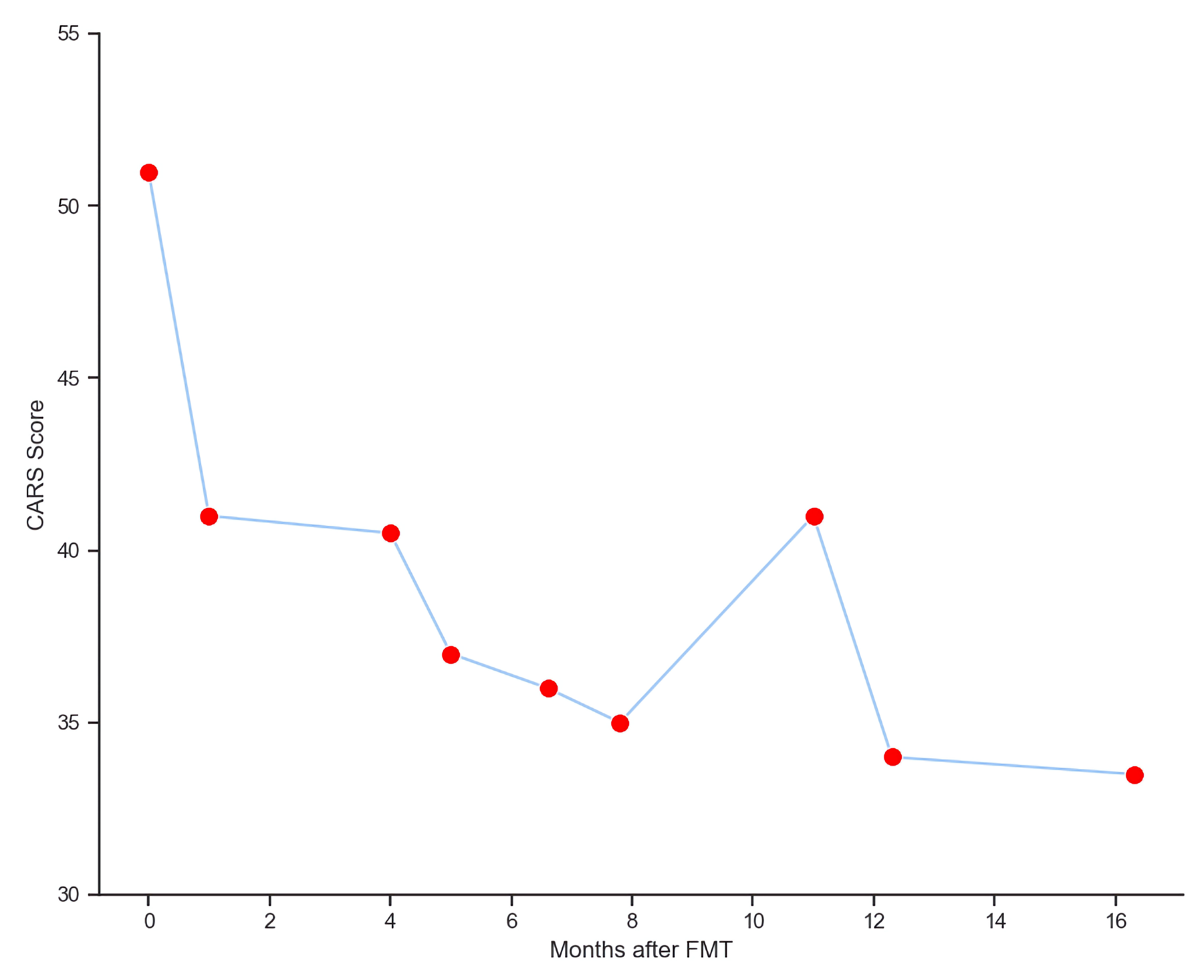 Click for large image | Figure 4. Improvements of CARS scores post FMT. CARS: Childhood Autism Rating Scale; FMT: fecal microbiota transplant. |
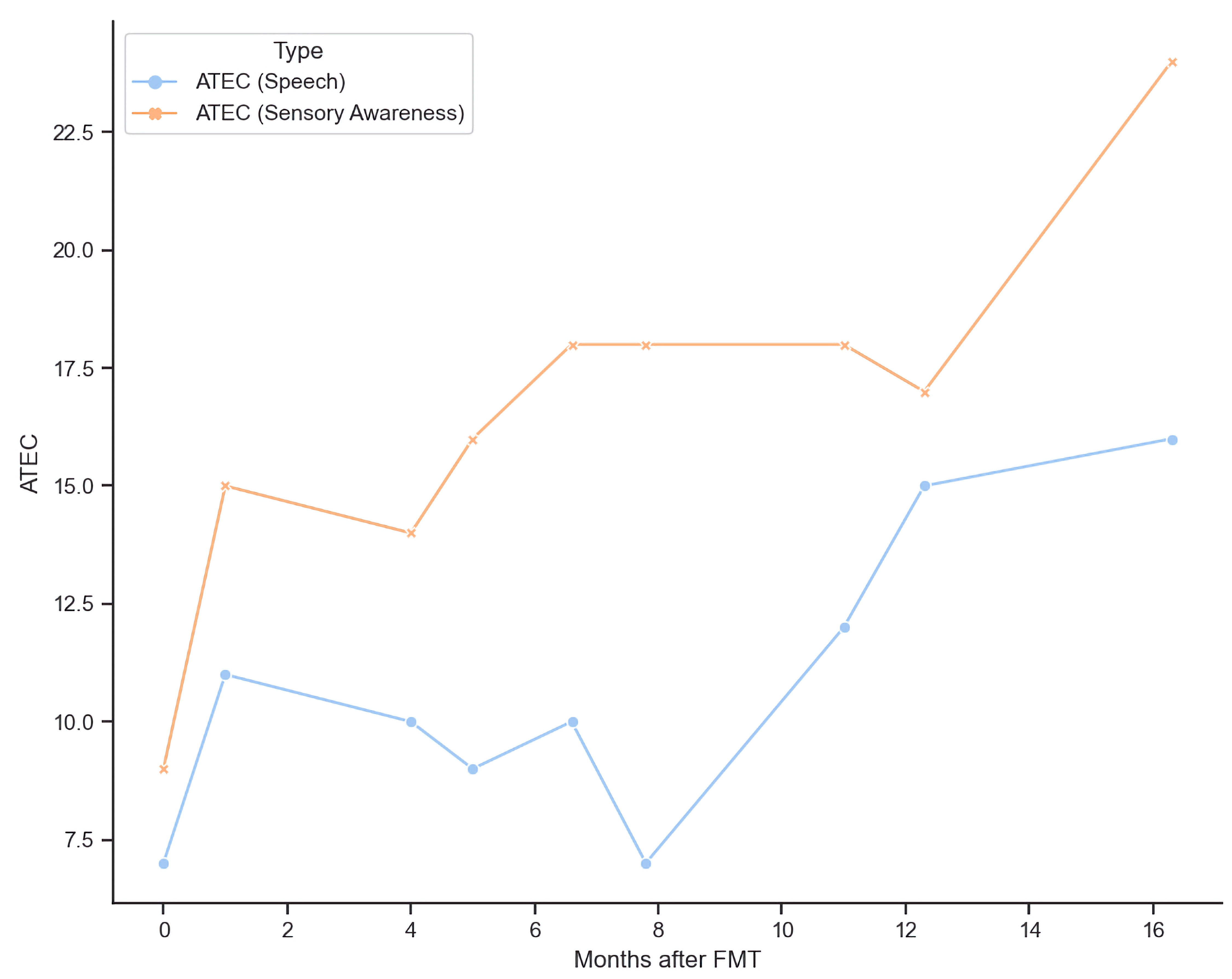 Click for large image | Figure 5. Improvements of ATEC scores post FMT. ATEC: Autism Treatment Evaluation Checklist; FMT: fecal microbiota transplant. |
The patients’ gastrointestinal symptoms also resolved, namely a marked decrease in bloating, constipation, and diarrhea. Prior to FMT, the patient used to have behaviors of banging his head and breaking his teeth, which completely disappeared following FMT therapy. Progressively, the patient improved speech to eight words, completed sentences with a board, paid attention in class and spelled words with a board. Caregivers reported that the patient had appropriate laughing episodes and was able to sit quietly in class.
| Discussion | ▴Top |
To the authors’ knowledge, this is the first report to demonstrate the use of familial FMT in an adolescent subject with ASD, resulting in notable improvements in gut microbiome composition during the 6 months that followed the FMT procedure. Specifically, by 6 months the subject experienced a marked reduction in relative abundance of phylum Proteobacteria, a marked increase in relative abundance of phylum Actinobacteria (particularly for genus Bifidobacterium), a disappearance of species Lactobacillus animalis, and a significant increase in Shannon index of bacterial diversity to a level that nearly matched the donor. Additionally, the subject who was previously non-verbal said his first two words (“mama” and “baba”) and became calmer at 1-month post-FMT. The subject’s CARS scores also decreased across time, although given the subject’s age, substantial changes were neither observed nor expected. While early in clinical assessment, this case demonstrates a successful application of FMT using a sibling donor to treat gut dysbiosis and alleviate neurobehavioral symptoms in an older subject with ASD. Given that improved ASD outcomes post-FMT are observed in younger patients, the authors hypothesize that the specific use of a familial FMT donor is a major reason why the subject in this case (e.g., a 19-year-old adolescent with severe ASD) experienced notable symptom improvement.
Prior research suggests that children with ASD tend to exhibit altered gut microflora compared to their typically developing counterparts, both in terms of overall diversity and specific composition [16-18]. While results are mixed, children with ASD appear to experience lower overall bacterial diversity, lower abundances of Akkermansia, Bacteroides, Bifidobacterium, Escherichia coli, and Enterococcus, higher abundances of Faecalibacterium and Lactobacillus, Ruminococcus, and Clostridium, and a reduced ratio between the phyla Bacteroidetes to Firmicutes [16-18]. Microbial dysbiosis may contribute to the pathogenesis and clinical presentation of ASD by disrupting brain development and function [16-18]. For example, increased levels of Faecalibacterium are strongly correlated with increased expression of interferon response factors 7 and 9, thus inducing proinflammatory pathways that dysregulate the immune system [16]. Increased levels of Clostridium may produce neurotoxins and yield alterations in phenylalanine metabolism that could contribute to ASD [16, 17]. Bifidobacteria produce lactic acid which suppresses the proliferation of pathogenic microbes in the epithelium and reduces inflammation; thus, reduced Bifidobacterium may contribute to the development of ASD via the loss of an important anti-inflammatory protective mechanism [16, 17]. Imbalances in short chain fatty acid levels, resulting from altered fermentation capacity of a dysbiotic gut, may have a negative effect on the biosynthesis of neurotransmitters and cellular energy production in the central and autonomic nervous systems, thus contributing further to the pathophysiology of ASD [17]. This is the first study we are aware of to show a specific relationship between Lactobacillus animalis and ASD. Further investigation is warranted to determine if this particular gut microbe plays a role in the presentation of ASD or other neurological disorders.
Improvements in gut microbiome composition and ASD symptoms following FMT are becoming increasingly evident in the literature, particularly since it is known that ASD patients suffer from gastrointestinal symptoms [39]. Ward et al [31] conducted a case series involving eight subjects ages 2 - 12 years and one subject who was aged 21 years, finding that FMT yielded more notable improvements in ASD symptoms for the younger subjects, but not the 21-year-old subject. Kang et al [32] conducted an open-label study involving 18 subjects ages 7 - 16 years who underwent 2 weeks of vancomycin treatment followed by 7 - 8 weeks of daily FMT administration, along with an 8-week post-FMT observation period. The FMT protocol yielded significant improvements in gut microbial diversity, including increased abundances of Bifidobacterium, Prevotella, and Desulfovibrio, which persisted at 8 weeks post-FMT [32]. Core ASD symptoms also remained improved at 8 weeks post-FMT and were accompanied by an average increase in developmental age of 1.4 years [32]. The authors reassessed this cohort of 18 subjects 2 years later and found that increased abundances of Bifidobacterium and Prevotella persisted, ASD core symptoms had continued to improve, and the average total increase in developmental age was 2.5 years [23]. Remarkably, the authors noted that 83% of the subjects were rated as having severe ASD prior to FMT, whereas at 2 years post-FMT, 17% were rated as having severe ASD, and 44% were now below the diagnostic cutoff for ASD [23, 32]. Li et al [22] investigated the effects of a 4-week FMT protocol followed by 8 weeks of observation in 18 subjects ages 3 - 17 years, noting a shift in the subjects’ post-FMT microbiome profile to resemble that of a control group of 16 age- and sex-matched typically developing children, along with improved neurobehavioral symptoms and social skills.
Although no published work has specifically reported on familial FMT in the context of ASD, three previous studies have examined post-FMT outcomes in subjects with gastrointestinal disease using donors who were blood relatives of the recipients. Shimizu et al [40] published a case report of an 11-year-old female with corticosteroid-dependent ulcerative colitis (UC), who was successfully treated using repeated FMT, in which her father was the donor. The subject experienced a marked shift in her gut microbiome profile pre- to post-FMT, characterized by a decrease in phylum Proteobacteria and an increase in Bacteroides, Acidaminococcus, Escherichia, Faecalibacterium, and Eubacterium [40]. In addition, the subject was able to successfully taper her corticosteroid dosage over time, achieving disease remission [40]. Okahara et al evaluated the long-term efficacy of FMT following triple antibiotic therapy in adult subjects with mild-to-severe UC, using subject spouses and adult relatives as donors, finding that disease remission rates were higher post-FMT for subjects who utilized sibling donors [41]. Dow et al [42] described a case report of a 21-month-old female with Pompe disease and B-cell immunodeficiency with recurrent Clostridium difficile infection (CDI), who was successfully treated with FMT, in which her mother was the donor. The subject experienced a complete resolution of symptoms and remained free of CDI at 5 years post-FMT. The authors of these studies suggest that healthy family members may provide an efficacious avenue for FMT donation, particularly for children, and that healthy siblings may possess a gut microbiome that reflects the pre-disease microbiome of their sibling with autism [40-42]. In the environment of UC, genetic and immunologic factors may influence stabilization of the gut microbiome, and healthy siblings may possess the desirable taxonomic profile needed to achieve long-term disease maintenance in their sibling FMT recipients [40, 41].
Mechanisms underlying the effectiveness of FMT in improving ASD outcomes continue to be investigated and appear to involve modulation of the gut-brain axis, which may improve epithelial barrier integrity and influence the activity of neurotransmitters such as 5-hydroxytryptamine, gamma-aminobutyric acid (GABA), and dopamine to regulate mood, behavior, and neurodevelopment [23, 35]. Limitations of the study, as mentioned earlier, is that this is only one ASD case treated, more ADS cases need to be treated via familial FMT to validate these results. Further investigation is warranted to determine if blood relatives of patients with ASD possess a specific desirable taxonomic microflora profile that is more likely to successfully engraft and enhance these underlying mechanisms, thus providing heightened improvements in gut microbiome composition and ASD clinical symptoms in their FMT recipient relatives.
Learning points
The use of FMT to normalize the gut microbiome and alleviate ASD symptoms for the subject in this report is consistent with previous evidence. Although this is only a preliminary report of a single case, the improvement in gut microbiome composition and clinical symptoms following FMT using a typically developing sibling donor is a novel finding and may suggest that older ASD subjects with a more severe clinical presentation could particularly benefit from this approach. Current treatment approaches for ASD are limited; thus, development of treatment modalities that target underlying medical and pathophysiological comorbidities associated with ASD is needed [2]. Familial FMT may be one powerful avenue for delivering precise medical care to subjects with ASD to support their unique needs and improve their functional outcomes, provided the donor’s microbiome is properly vetted.
Acknowledgments
None to declare.
Financial Disclosure
This research received no external funding.
Conflict of Interest
Sabine Hazan is CEO of Ventura Clinical Trials and ProgenaBiome and owner of Microbiome Research Foundation. Sabine Hazan declares that she has a pecuniary interest in Topelia LLC USA. She has patents relevant to microbiome testing and fecal matter transplant. Drs. Haroon and Jordan performed psychological and neurological evaluation on the case and have no conflict of interest to disclose. Dr Walker has no conflict of interest.
Informed Consent
Written informed consent was obtained from the subject’s caregiver.
Author Contributions
Conceptualization: SH and SJ. Methodology: SH. Investigation: JH and SJ. Writing - original draft preparation: SH, JH and SJW. Writing - review and editing: SJW. Supervision: SH. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- CDC. Basics About Autism Spectrum Disorder (ASD). https://www.cdc.gov/ncbddd/autism/facts.html. 2022.
- Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull. 2017;33(2):183-193.
doi pubmed pmc - Hyman SL, Levy SE, Myers SM, Council On Children With Disabilities, Section On Developmental Behavioral, Pediatrics. Identification, evaluation, and management of children with autism spectrum Disorder. Pediatrics. 2020;145(1):e20193447.
doi pubmed - Elsabbagh M. Linking risk factors and outcomes in autism spectrum disorder: is there evidence for resilience? BMJ. 2020;368:l6880.
doi pubmed - Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, Furnier SM, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 Sites, United States, 2018. MMWR Surveill Summ. 2021;70(11):1-16.
doi pubmed pmc - Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Association. 2013.
- Styles M, Alsharshani D, Samara M, Alsharshani M, Khattab A, Qoronfleh MW, Al-Dewik NI. Risk factors, diagnosis, prognosis and treatment of autism. Front Biosci (Landmark Ed). 2020;25(9):1682-1717.
doi pubmed - Nadeem MS, Al-Abbasi FA, Kazmi I, Murtaza BN, Zamzami MA, Kamal MA, Arif A, et al. Multiple risk factors: a challenge in the management of autism. Curr Pharm Des. 2020;26(7):743-754.
doi pubmed - Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin Microbiol Rev. 2022;35(1):e0033820.
doi pubmed pmc - Wang Q, Luo Y, Ray Chaudhuri K, Reynolds R, Tan EK, Pettersson S. The role of gut dysbiosis in Parkinson's disease: mechanistic insights and therapeutic options. Brain. 2021;144(9):2571-2593.
doi pubmed - Parodi B, Kerlero de Rosbo N. The gut-brain axis in multiple sclerosis. Is its dysfunction a pathological trigger or a consequence of the disease? Front Immunol. 2021;12:718220.
doi pubmed pmc - Noto D, Miyake S. Gut dysbiosis and multiple sclerosis. Clin Immunol. 2022;235:108380.
doi pubmed - Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K, Hirayama M. Progression of Parkinson's disease is associated with gut dysbiosis: Two-year follow-up study. PLoS One. 2017;12(11):e0187307.
doi pubmed pmc - Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer's disease. J Alzheimers Dis. 2017;58(1):1-15.
doi pubmed - Liu S, Gao J, Zhu M, Liu K, Zhang HL. Gut microbiota and dysbiosis in Alzheimer's disease: implications for pathogenesis and treatment. Mol Neurobiol. 2020;57(12):5026-5043.
doi pubmed pmc - Xu M, Xu X, Li J, Li F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatry. 2019;10:473.
doi pubmed pmc - Srikantha P, Mohajeri MH. The possible role of the Microbiota-Gut-Brain-Axis in autism spectrum disorder. Int J Mol Sci. 2019;20(9):2115.
doi pubmed pmc - Fattorusso A, Di Genova L, Dell'Isola GB, Mencaroni E, Esposito S. Autism spectrum disorders and the gut microbiota. Nutrients. 2019;11(3):521.
doi pubmed pmc - Hazan S. Rapid improvement in Alzheimer's disease symptoms following fecal microbiota transplantation: a case report. J Int Med Res. 2020;48(6):300060520925930.
doi pubmed pmc - Zebrowska P, Laczmanska I, Laczmanski L. Future directions in reducing gastrointestinal disorders in children with ASD using fecal microbiota transplantation. Front Cell Infect Microbiol. 2021;11:630052.
doi pubmed pmc - Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98.
doi pubmed pmc - Li N, Chen H, Cheng Y, Xu F, Ruan G, Ying S, Tang W, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell Infect Microbiol. 2021;11:759435.
doi pubmed pmc - Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9(1):5821.
doi pubmed pmc - Kuai XY, Yao XH, Xu LJ, Zhou YQ, Zhang LP, Liu Y, Pei SF, et al. Evaluation of fecal microbiota transplantation in Parkinson's disease patients with constipation. Microb Cell Fact. 2021;20(1):98.
doi pubmed pmc - Al KF, Craven LJ, Gibbons S, Parvathy SN, Wing AC, Graf C, Parham KA, et al. Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial. Mult Scler J Exp Transl Clin. 2022;8(2):20552173221086662.
doi pubmed pmc - Engen PA, Zaferiou A, Rasmussen H, Naqib A, Green SJ, Fogg LF, Forsyth CB, et al. Single-arm, non-randomized, time series, single-subject study of fecal microbiota transplantation in multiple sclerosis. Front Neurol. 2020;11:978.
doi pubmed pmc - Xu MQ, Cao HL, Wang WQ, Wang S, Cao XC, Yan F, Wang BM. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol. 2015;21(1):102-111.
doi pubmed pmc - Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm. 2018;5(4):e459.
doi pubmed pmc - Borody T, Leis S, Campbell J, Torres M, Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Official Journal of the American College of Gastroenterology | ACG. 2011;106:942.
- Borody T, Rosen D, Torres M, Campbell J, Nowak A. Myoclonus-dystonia affected by GI microbiota? Official Journal of the American College of Gastroenterology | ACG. 2011;106:940.
- Ward L, O’Grady HM, Wu K, Cannon K, Workentine M, Louie T. Combined oral fecal capsules plus fecal enema as treatment of late-onset autism spectrum disorder in children: report of a small case series. Open Forum Infect Dis. 2016;3(Suppl 1):S599.
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10.
doi pubmed pmc - Zhang J, Zhu G, Wan L, Liang Y, Liu X, Yan H, Zhang B, et al. Effect of fecal microbiota transplantation in children with autism spectrum disorder: A systematic review. Front Psychiatry. 2023;14:1123658.
doi pubmed pmc - Huang HL, Xu HM, Liu YD, Shou DW, Chen HT, Nie YQ, Li YQ, et al. First Application of fecal microbiota transplantation in adult asperger syndrome with digestive symptoms - a case report. Front Psychiatry. 2022;13:695481.
doi pubmed pmc - Hazan S, Dave S, Papoutsis AJ, Barrows BD, Borody TJ. Successful Bacterial Engraftment Identified by Next-Generation Sequencing Predicts Success of Fecal Microbiota Transplant for Clostridioides difficile. Gastroenterology Res. 2021;14(5):304-309.
doi pubmed pmc - Papoutsis A, Borody T, Dolai S, Daniels J, Steinberg S, Barrows B, Hazan S. Detection of SARS-CoV-2 from patient fecal samples by whole genome sequencing. Gut Pathog. 2021;13(1):7.
doi pubmed pmc - Hazan S, Stollman N, Bozkurt HS, Dave S, Papoutsis AJ, Daniels J, Barrows BD, et al. Lost microbes of COVID-19: Bifidobacterium, Faecalibacterium depletion and decreased microbiome diversity associated with SARS-CoV-2 infection severity. BMJ Open Gastroenterol. 2022;9(1):e000871.
doi pubmed pmc - Grissom M. Childhood autism rating scales. In: Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011. p. 553-554.
- Lasheras I, Real-Lopez M, Santabarbara J. Prevalence of gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. An Pediatr (Engl Ed). 2023;99(2):102-110.
doi pubmed - Shimizu H, Arai K, Abe J, Nakabayashi K, Yoshioka T, Hosoi K, Kuroda M. Repeated fecal microbiota transplantation in a child with ulcerative colitis. Pediatr Int. 2016;58(8):781-785.
doi pubmed - Okahara K, Ishikawa D, Nomura K, Ito S, Haga K, Takahashi M, Shibuya T, et al. Matching between donors and ulcerative colitis patients is important for long-term maintenance after fecal microbiota transplantation. J Clin Med. 2020;9(6):1650.
doi pubmed pmc - Dow DE, Seed PC. Clostridium difficile cure with fecal microbiota transplantation in a child with Pompe disease: a case report. J Med Case Rep. 2018;12(1):112.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


