| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 4-5, May 2024, pages 72-77
Recurrent Takotsubo Cardiomyopathy due to Pheochromocytoma Managed With Venoarterial Extracorporeal Membrane Oxygenation
Georgia Karmiotia, Stefanos Sakellaropoulosb, Inan Sarikayac, Antonia Kastorisd, Christina Hadjiloukad, Christos Efsevioud, Michael Myrianthefsa, Andreas Mitsisa, e
aCardiology Department, Nicosia General Hospital, Nicosia, Cyprus
bDepartment of Internal Medicine, Cardiology Clinic, Kantonsspital Baden, Baden, Switzerland
cPraxis Gruppe Wurenlingen, Internal Medicine, Wurenlingen, Switzerland
dIntensive Care Unit, Nicosia General Hospital, Nicosia, Cyprus
eCorresponding Author: Andreas Mitsis, Cardiology Department, Nicosia General Hospital, Nicosia 2029, Cyprus
Manuscript submitted February 20, 2024, accepted April 19, 2024, published online May 2, 2024
Short title: VA ECMO Support of Takotsubo Cardiomyopathy
doi: https://doi.org/10.14740/jmc4195
| Abstract | ▴Top |
Pheochromocytoma-induced Takotsubo cardiomyopathy is a rare but life-threatening condition, caused by excessive plasma catecholamine levels, resulting in acute myocardial dysfunction. Clinical presentation includes a rapid development of heart failure due to regional wall motion abnormalities (most commonly affecting all mid to apical left ventricle (LV) wall segments) causing the “octopus-trap-like” LV shape. A 45-year-old female patient presented with acute cardiogenic shock of non-ischemic etiology. Her past medical history included a similar episode, which was followed by full recovery, but at this admission she required hemodynamic support with venoarterial extracorporeal membrane oxygenation. The systolic function was restored, and further investigation revealed high 24-h urine metanephrine levels and a mass of the left adrenal gland, leading to the diagnosis of pheochromocytoma. After treatment with firstly alpha-blockers and then beta-blockers, the pheochromocytoma was surgically removed.
Keywords: Takotsubo cardiomyopathy; Cardiogenic shock; Pheochromocytoma; Venoarterial extracorporeal membrane oxygenation; Heart failure
| Introduction | ▴Top |
Takotsubo cardiomyopathy (TC) is an acute, stress-induced condition that often results in temporary failure of the left ventricle (LV), characterized by regional wall motion abnormalities, particularly severe hypokinesia of the apical and mid-ventricular wall segments [1]. Recent descriptions have identified less common variants affecting the mid or basal ventricular wall segments, termed “reverse TC” [2]. Despite extensive speculation, the pathophysiology of TC remains unclear [3]. Pheochromocytoma-induced Takotsubo cardiomyopathy (PiTC) has been described in the literature as a rare and often life-threatening severe cardiomyopathy, due to its rather usual complication with cardiogenic shock and no single pattern of ventricular dysfunction [4].
In this case report, we present a 45-year-old female patient with TC which was complicated with cardiogenic shock. The patient experienced a similar episode of TC 1 year earlier, from which she has completely recovered. The second episode though, due to the severe deterioration of the hemodynamic status, required intubation and venoarterial extracorporeal membrane oxygenation (VA ECMO). Early recovery and VA ECMO weaning gave the necessary time for a complete diagnostic workup that revealed a left-sided adrenal pheochromocytoma as the cause of the acute heart failure. After initial support and PiTC management, patient was treated with surgical removal of the pheochromocytoma. This made clear that the recurrence of the TC was due to the untreated pheochromocytoma.
The purposes of this manuscript are firstly to describe the entity of PiTC and the specific characteristics of this sub-category of TC, and secondly to demonstrate that the use of VA ECMO in this patient with acute cardiogenic shock might be lifesaving, until a complete diagnosis of the underlying condition is made.
| Case Report | ▴Top |
Investigations
A 45-year-old Eastern European female patient presented at a tertiary care hospital with complaints of sudden-onset dyspnea, chest pain radiating to the back, and nausea. She had a history of epilepsy, was treated since her adolescence, and was hospitalized 1 year prior at a district hospital for reported cardiogenic shock related to a Takotsubo-like syndrome. During that episode, she underwent intubation for 5 days, but she had fully recovered. On the current admission, she exhibited hypertension and low oxygen saturation, with elevated troponin levels. The initial electrocardiogram (ECG) displayed ST-elevation in leads V1-V2 and ST-depression in leads II and III. Consequently, she was urgently referred to a tertiary care center for emergency coronary angiography. Treatment commenced with dual antiplatelet therapy comprising aspirin and ticagrelor, a vasodilator infusion (isosorbide dinitrate), intravenous diuretic (furosemide), and oxygen therapy via a venturi mask.
Diagnosis
Coronary angiography revealed no significant atherosclerotic changes in the coronary arteries (Fig. 1a), while left ventriculography demonstrated markedly reduced LV function, characterized by severe hypokinesia of the basal and mid wall segments and mild hypokinesia of the apical segments (Fig. 1b), without evidence of mitral valve regurgitation or a gradient between the LV and aorta. Concurrent echocardiography confirmed the severe impairment of LV function, with severe hypokinesia from the basal to mid segments, suggesting a reverse TC as the preliminary diagnosis (Fig. 2a).
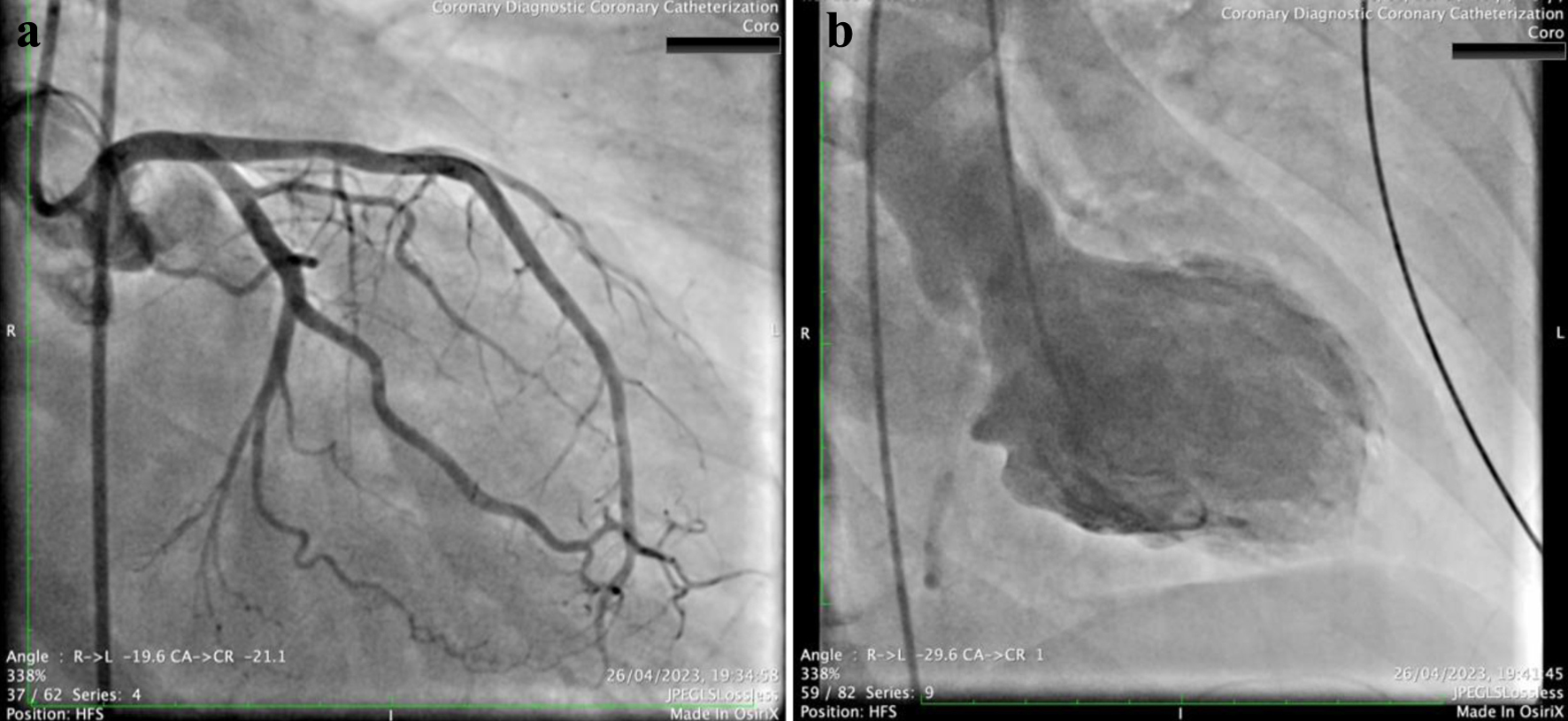 Click for large image | Figure 1. (a) Coronary angiography of the left coronary system in right anterior oblique caudal view, showing no signs of significant coronary artery disease. (b) Left ventriculography in right anterior oblique 30°, showing a severely impaired left ventricle function with balloon-like left ventricle formation, due to severe hypokinesia of basal to mid left ventricle wall segments and mild hypokinesia of apical left ventricle wall segments. |
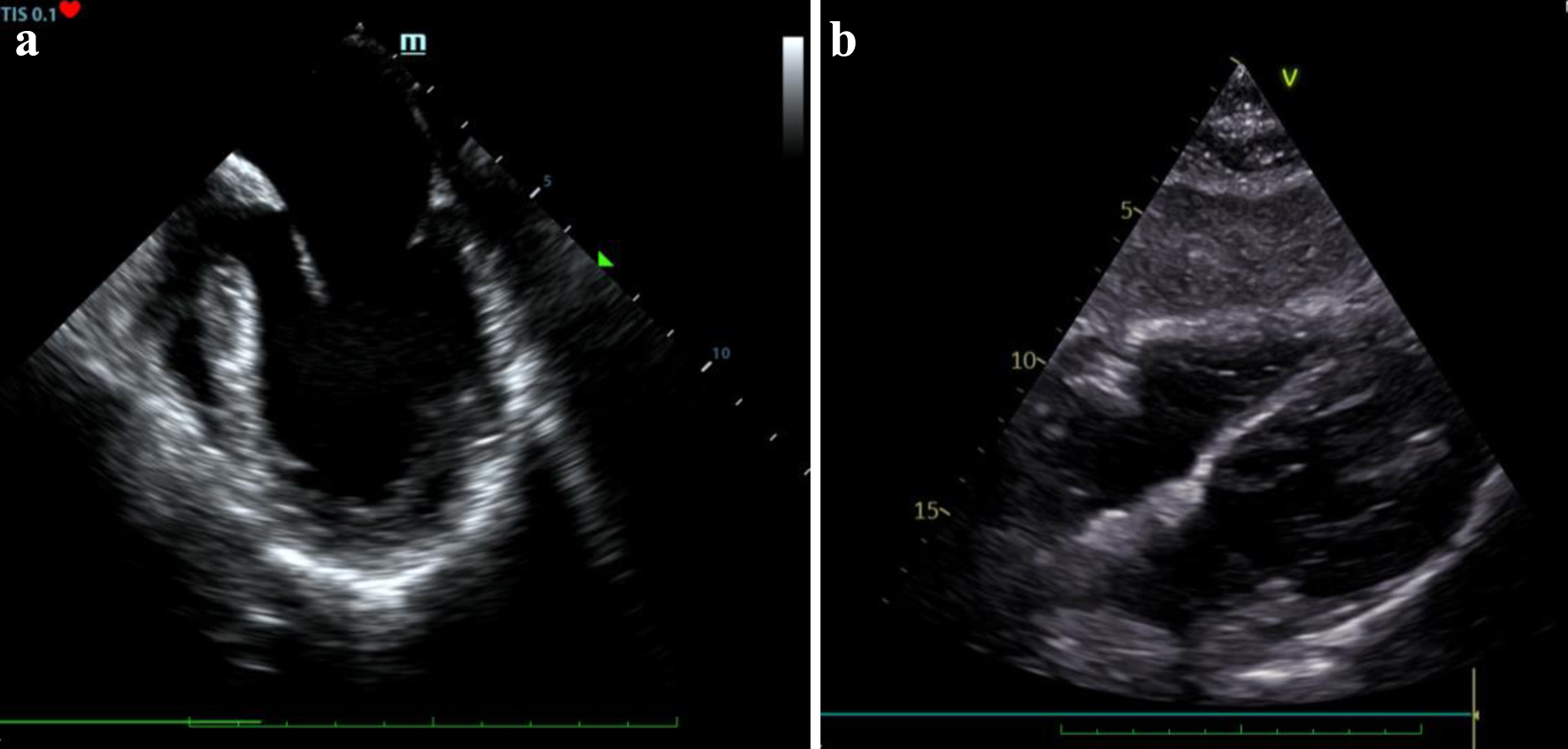 Click for large image | Figure 2. (a) Echocardiography view of severely impaired left ventricle systolic function, RV size within normal limits and no pericardial effusion (day 0). (b) Follow-up echocardiography showing an improved left ventricle systolic function and no pericardial effusion (day 5). |
Treatment
Given the patient’s critical condition at the time with worsening respiratory distress and deteriorating hemodynamic status, the patient was intubated and placed on mechanical ventilation. The rapid hemodynamic deterioration with increasing needs for both vasopressor and inotropic support, with increasing lactate levels, worsening metabolic acidosis and declining cardiac function prompted support by VA ECMO.
VA ECMO placement was achieved through peripheral access with the assistance of echocardiography. Venous access for drainage was established in the right femoral vein, with a 25 French, 55 cm cannula, whereas arterial access was achieved via the left femoral artery, with a 19 French, 15 cm cannula after revascularization of the left lower extremity with 8 French cannula (V25/55fr-A19/15fldt). The patient was placed on a Cardiohelp HLS system with temperature regulation. She required initially 3 to 3.4 L/min of blood flow within the first 24 h of placement, achieving rapidly decreasing needs for both vasopressor and inotropic support. Invasive hemodynamic monitoring was established with a Swan-Ganz catheter in the right jugular vein.
She was successfully weaned off the ECMO after 48 h with an impressive recovery of the LV with an almost normal LV function established via transesophageal echocardiography. The arterial cannula was removed in the operating theater without adverse events. Anticoagulation was achieved with ease with unfractionated heparin infusion. No adverse events or ECMO-related complications were observed during the ECMO run.
During that time, further investigation included daily laboratory testing that was unremarkable, negative toxicology screening, negative microbial bronchoalveolar lavage cultures, negative respiratory virus polymerase chain reaction (PCR) panel test and negative severe acute respiratory syndrome coronavirus 2 PCR testing. Tuberculosis and Pneumocystis carinii testing were also negative. Pregnancy testing was negative and thyroid hormone levels were normal. Vanillylmandelic acid levels in the urine were tested and were found positive (310 mg/24 h with a normal range < 11/24 h mg). Echocardiography showed an improved LV systolic function (left ventricular ejection fraction (LVEF): about 50%) with only mild hypokinesia of basal LV wall segments and mild concentric left ventricular hypertrophy (interventricular septum thickness 13 mm, left ventricular posterior wall 12 mm), without any significant valvulopathy, no pericardial effusion and no thrombus in the left atrium (Fig. 2b).
The patient was extubated after 6 days (4 days after ECMO removal), needing nasal oxygen supply for the next 3 days. With the patient hemodynamically stable, fully alert, and oriented, further investigation for secondary hypertension and stress triggers was initiated. A computed tomography of chest and abdomen revealed small bilateral pleural effusions and ground glass opacity areas mainly in the left lower lobe, a small intra-abdominal effusion, a right ovarian cyst and finally a contrast-enhanced mass (4.1 × 3.0 × 3.1 cm) on the left adrenal gland (Fig. 3a). Further laboratory testing with urine catecholamines (urine epinephrine was measured 40 µg/24 h and urine norepinephrine measured 150 µg/24 h) and urine metanephrines (613 µg/24 h) as well as magnetic resonance imaging of the adrenal glands confirmed the diagnosis of pheochromocytoma (Fig. 3b). Meanwhile, on day 14, a new evaluation with transthoracic echocardiography confirmed that systolic function of the LV had fully recovered, with an LVEF of about 60-65%, normal size of all heart chambers, a mild mitral valve regurgitation and moderate tricuspid valve regurgitation with mild pulmonary hypertension (right ventricular systolic pressure: about 58 mm Hg).
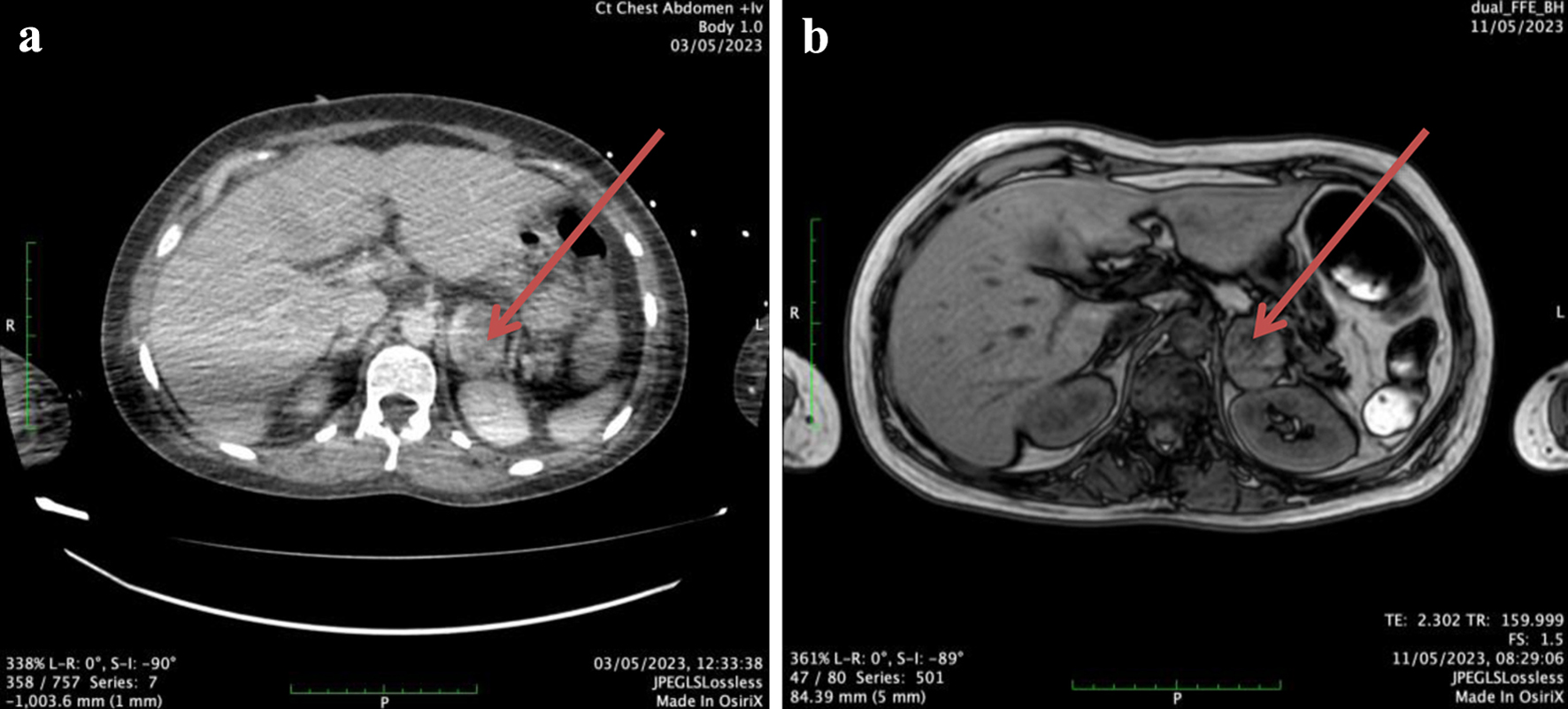 Click for large image | Figure 3. (a) A computed tomography scan cut showing a rather large (> 4 cm) lesion (red arrow) of the left adrenal gland with areas of cystic degeneration and heterogeneous contrast uptake. (b) A magnetic resonance imaging scan cut showing an enlarged left adrenal gland (30 × 37 × 37 mm) (red arrow) with contrast uptake during the early systemic arterial phase which remains during all imaging phases - no early washout is seen. |
Follow-up and outcomes
After the diagnosis of pheochromocytoma was confirmed, further management of the patient included antihypertensive medical therapy, alpha-blockage for 15 days and then beta-blockage with for 15 days, before surgical removal of the left intra-adrenal pheochromocytoma that was successfully performed without adverse events, 1 month after diagnosis. The patient was then discharged hemodynamically stable, pending long-time follow-up.
| Discussion | ▴Top |
TC is characterized by a temporary balloon-like deformation of the LV apex, causing the ventricles to resemble a “tsubo” - a basket used by Japanese fishermen to trap octopuses (“tako”). Although the exact pathophysiology of TC remains elusive, it is widely believed to involve excessive sympathetic nervous system stimulation [5]. Predominantly affecting post-menopausal women, TC typically manifests with symptoms similar to acute coronary syndrome, including chest pain and sudden-onset dyspnea. These symptoms arise from LV regional wall motion abnormalities that span the territories of more than one coronary artery, accompanied by elevated cardiac biomarkers (such as troponin and creatine phosphokinase/creatine kinase-MB) and ECG changes, which may include ST-segment elevation or depression, T-wave inversion, and QT prolongation [1, 3, 5]. TC is distinguished from other cardiomyopathies by the transient nature of the heart failure and its triggers, which can be physical and/or emotional stressors, or neurological disorders, after excluding other causes such as infectious myocarditis. Notably, the presence of concurrent coronary artery disease does not preclude a TC diagnosis [3, 6].
TC may be triggered by various physical, emotional, even environmental factors; however, identifying a trigger is not always feasible, with 30% of patients remaining without trigger diagnosis [7]. Emotional stressors include emotional trauma (e.g., grief), conflict, fear or panic, anger, anxiety, embarrassment or even extreme positive feelings (e.g., winning the lottery). Physical stressors, commonly found in men, include extreme physical activity [1, 7]. Other triggers may include respiratory distress (e.g., asthma attack, pneumothorax), intra-abdominal infections such as pancreatitis, neurological emergencies, thyrotoxicosis, malignancies, pregnancy, drugs and alcohol and last but not least catecholamine crises caused by medication or endogenously by a pheochromocytoma [1].
PiTC has been described in literature as a rare and life-threatening severe cardiomyopathy often complicated with cardiogenic shock [8]. In a recent literature review, it is described that complications are nearly three times more likely in PiTC than any other cause of TC, with cardiogenic shock noted in nearly 26% of patients. From a pathophysiological viewpoint, the widely supported hypothesis is that significantly increased levels of plasma catecholamines cause an excessive sympathetic autonomous nervous system response, leading to acute myocardial injury [9]. This hypothesis is mainly supported by case reports and series where patients underwent a major emotional event or stressor (most commonly women of an average 65 to 70 years of age), including those requiring administration of catecholamines as treatment or those involving catecholamine-producing masses. The exact mechanism of excessive catecholamine-induced myocardial injury is not clear; however, suggested mechanisms include microcirculation dysfunction, spasm of multiple epicardial vessels, plaque rapture and direct catecholamine-induced cardiotoxicity on cardiomyocytes [4, 9].
When PiTC is compared to typical TC, several points differentiating PiTC are noted. Patients with PiTC seem to be slightly younger at age and the gap between men and women patients seems to be less than that of typical TC [10]. PiTC, amongst anaphylactic shock, antidepressant overdose and subarachnoid haemorrhage, is also more often expressed as reverse, rather than typical Takotsubo [11]. Complications, such as cardiogenic shock, are also more often in patients with PiTC; however, PiTC is rapidly suppressed, and LV systolic function seems to recover faster, indicating that patients in cardiogenic shock should be fully supported since the cause is surely and rapidly reversible [12]. Reverse Takotsubo is statistically more likely than classic Takotsubo, to result in cardiogenic shock and is also more often related to pheochromocytoma [13, 14].
Managing PiTC usually starts with treating the life-threatening TC, especially when cardiogenic shock is involved, before treating the underlying pheochromocytoma. Medical treatment usually includes beta-blockers or angiotensin-converting enzyme inhibitors and other heart failure medications; however, it is clearly imperative for healthcare providers to personalize treatment for each patient [15].
Cardiogenic shock due to TC could potentially be the first major clinical presentation of a pheochromocytoma, since other manifestations only include minor symptoms (sweating, headache, palpitations). Patients with cardiogenic shock due to TC may qualify for extracorporeal circulatory supportive interventions. VA ECMO is a form of temporary mechanical cardiopulmonary support that ensures tissue oxygenation and hemodynamic support in cases of acute cardiorespiratory failure [16]. Venous blood is drained through peripherally or centrally placed cannulas, with the help of a pump, where oxygenation through an oxygenator occurs and returned to the arterial circulation again through either peripherally or centrally placed cannulas. Indications for VA ECMO, other than non-ischemic or ischemic cardiogenic shock, include among others postoperative (post-transplant) patients, bridge to therapy (e.g., patients awaiting left ventricular assist device), cardiac arrest and prophylaxis during high-risk percutaneous cardiac interventions. It has been previously reported in the literature that in severe cases (as reported in our patient) with reversible cardiogenic shock due to pheochromocytoma crisis, the initiation of VA ECMO has been not only necessary but also lifesaving [17, 18].
Conclusion
This female patient presented with signs and symptoms of cardiogenic shock due to acute heart failure and was diagnosed with TC which required for her to be placed on VA ECMO for the first days of her treatment in order to survive the life-threatening acute phase of the syndrome. Further assessment and investigation led to the diagnosis of unilateral pheochromocytoma which was surgically removed after indicated medical treatment. What is interesting about this 45-year-old patient is the ST elevation myocardial infarction-like presentation of a recurrent acute heart failure syndrome with Takotsubo-specific regional wall motion abnormalities in the absence of hypertensive crisis. This case report reminds us - intensive care/cardiac care physicians - how patients, especially young women, presenting with cardiogenic shock and TC, should be investigated for further underlying causes and comorbidities which may often go undiagnosed.
Learning points
TC should be considered in the differential diagnosis for patients presenting with myocardial infarction-like signs and symptoms, when LV imaging shows wall motion abnormalities of all apical segments and coronary angiography shows no significant coronary artery disease. TC is mostly reversible, and this may confirm the diagnosis in follow-up.
Any patient presenting with TC, after initial support and management, should be further investigated for an underlying cause. Patients presenting with recurrent Takotsubo-like syndrome, should be thoroughly investigated for triggering underlying chronic diseases. Pheochromocytoma should be considered for those patients.
VA ECMO is often indicated as a bridging therapy in patients presenting with cardiogenic shock due to TC, as part of the initial acute heart failure management, since heart failure is, by definition, reversible.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
GK and AM participated in patient care, writing of the case report, revisions, and submission process. AK, CH, CE, and MM participated in patient care, writing of the case report and revisions. SS and IS participated in writing of the case reports and revisions.
Data Availability
All data in our report were obtained from the patient’s hospitalization. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Dias A, Nunez Gil IJ, Santoro F, Madias JE, Pelliccia F, Brunetti ND, Salmoirago-Blotcher E, et al. Takotsubo syndrome: state-of-the-art review by an expert panel - Part 1. Cardiovasc Revasc Med. 2019;20(1):70-79.
doi pubmed - Awad HH, McNeal AR, Goyal H. Reverse Takotsubo cardiomyopathy: a comprehensive review. Ann Transl Med. 2018;6(23):460.
doi pubmed pmc - Dias A, Nunez Gil IJ, Santoro F, Madias JE, Pelliccia F, Brunetti ND, Salmoirago-Blotcher E, et al. Takotsubo syndrome: state-of-the-art review by an expert panel - Part 2. Cardiovasc Revasc Med. 2019;20(2):153-166.
doi pubmed - Aw A, de Jong MC, Varghese S, Lee J, Foo R, Parameswaran R. A systematic cohort review of pheochromocytoma-induced typical versus atypical Takotsubo cardiomyopathy. Int J Cardiol. 2023;371:287-292.
doi pubmed - Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971.
doi pubmed pmc - Napp LC, Cammann VL, Jaguszewski M, Szawan KA, Wischnewsky M, Gili S, Knorr M, et al. Coexistence and outcome of coronary artery disease in Takotsubo syndrome. Eur Heart J. 2020;41(34):3255-3268.
doi pubmed - Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602-609.
doi pubmed pmc - Mitsis A, Hadjilouka C, Skarpari M, Myrianthefs M. An unusual case of pheochromocytoma mimicking both acute coronary syndrome and central nervous system infection. Case report and literature review. Hellenic J Cardiol. 2017;58(5):372-377.
doi pubmed - Sharkey SW, McAllister N, Dassenko D, Lin D, Han K, Maron BJ. Evidence that high catecholamine levels produced by pheochromocytoma may be responsible for Takotsubo cardiomyopathy. Am J Cardiol. 2015;115(11):1615-1618.
doi pubmed - Riester A, Weismann D, Quinkler M, Lichtenauer UD, Sommerey S, Halbritter R, Penning R, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764.
doi pubmed - Zhang R, Gupta D, Albert SG. Pheochromocytoma as a reversible cause of cardiomyopathy: analysis and review of the literature. Int J Cardiol. 2017;249:319-323.
doi pubmed - Mitsis A, Kyriakou M, Avraamides P. Pheochromocytoma-induced Takotsubo cardiomyopathy: beware of the rapid recovery. Hellenic J Cardiol. 2024.
doi pubmed - Ahmad H, Jannat H, Khan U, Ahmad N. Reverse Takotsubo cardiomyopathy triggered by undiagnosed right adrenal pheochromocytoma: a rare occurrence. Cureus. 2023;15(6):e40924.
doi pubmed pmc - Kim S, Yu A, Filippone LA, Kolansky DM, Raina A. Inverted-Takotsubo pattern cardiomyopathy secondary to pheochromocytoma: a clinical case and literature review. Clin Cardiol. 2010;33(4):200-205.
doi pubmed pmc - Yalta K. Pheochromocytoma-induced takotsubo syndrome: what does an intensivist need to know? Anaesthesiol Intensive Ther. 2023;55(4):315-316.
doi pubmed pmc - Banga S, Challa A, Patel AR, Singh S, Emani VK. The patient selection criteria for veno-arterial extracorporeal mechanical oxygenation. Cureus. 2019;11(9):e5709.
doi pubmed pmc - Hekimian G, Kharcha F, Brechot N, Schmidt M, Ghander C, Lebreton G, Girerd X, et al. Extracorporeal membrane oxygenation for pheochromocytoma-induced cardiogenic shock. Ann Intensive Care. 2016;6(1):117.
doi pubmed pmc - Fennell D, Miller C, Ludgate S, Conneely J, O’Brien S, Conrick-Martin I, Hastings J, et al. Two cases of cardiomyopathy associated with phaeochromocytoma successfully managed with veno-arterial extracorporeal membrane oxygenation (V-A ECMO). Endocrinol Diabetes Metab Case Rep. 2023;2023(2):22-0392.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


