| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 12, December 2023, pages 413-418
Catheter Ablation of Left Ventricular Summit Ectopies in Left Ventricular Noncompaction
Division of Electrophysiology, Department of Adult Cardiology, Philippine Heart Center, Quezon City 1100, Philippines
Manuscript submitted November 28, 2023, accepted December 15, 2023, published online December 29, 2023
Short title: Catheter Ablation in LVNC
doi: https://doi.org/10.14740/jmc4178
| Abstract | ▴Top |
Left ventricular noncompaction (LVNC) is a rare disorder and the true prevalence is largely unknown. Its clinical presentation is highly variable from being asymptomatic to the presence of heart failure, thromboembolic events, arrhythmias, and even risk of sudden cardiac death. A 37-year-old woman presented with frequent and symptomatic premature ventricular complexes (PVCs) and reduced left ventricular systolic function due to LVNC cardiomyopathy. The PVCs were refractory to medical therapy and the patient underwent successful ablation of the left ventricular summit PVCs. There was no recurrence of the PVCs at 6 months follow-up. This case report adds to the growing evidence of the efficacy and safety of performing radiofrequency ablation of ventricular arrhythmias (VAs) refractory to medical therapy in patients with LVNC. The different mechanisms of the VAs and therapeutic options are also reviewed.
Keywords: Ventricular arrhythmia; Left ventricular noncompaction; Cardiomyopathy; Radiofrequency ablation; 3D electroanatomic mapping
| Introduction | ▴Top |
Left ventricular noncompaction (LVNC), first described by Grant in 1926, was only classified as a primary genetic cardiomyopathy by the American Heart Association in 2006 [1, 2]. It is characterized by the distinctive appearance of the left ventricular myocardium with two distinct layers: a compact layer and a noncompact layer with prominent trabeculations and deep intertrabecular recesses (creating the “spongy” appearance) predominantly involving the apical portion of the left ventricle (LV) chamber. These abnormalities result from arrest in the normal embryonic development of the myocardium in the first trimester. The European Society of Cardiology on the other hand does not consider LVNC as a cardiomyopathy, but as a phenotypic trait that can occur in isolation or in association with other cardiac anomalies [3].
LVNC is a rare disorder. Although the true prevalence is largely unknown, a range of 0.014% to 1.3% has been reported [4]. Its clinical expression is highly variable. Patients may present with no symptom or with frank heart failure, systemic thromboembolic events, arrhythmias, and even sudden death [5]. In this case report, a 37-year-old woman presented with LVNC associated with frequent, symptomatic premature ventricular complexes (PVCs) and impaired LV systolic function who underwent successful radiofrequency catheter ablation.
| Case Report | ▴Top |
Investigations
The case is of a 37-year-old female overseas worker who 3 years before admission started to experience intermittent palpitation described as “skip beats” with no other associated symptoms. There was no family history of cardiac diseases and unexplained sudden death. The patient was evaluated in a hospital overseas and 24-h ambulatory electrocardiogram (ECG) showed isolated monomorphic PVCs (< 1% burden). Transthoracic echocardiogram showed normal-sized LV but with global hypokinesia and depressed ejection fraction of 35%. There was noncompaction in the apex. Cardiac magnetic resonance imaging (MRI) showed prominent left ventricular trabeculations from apex to base with 3:1 ratio of the noncompacted to the compacted myocardium. This finding in the MRI is diagnostic as described by Petersen et al in 2005 that a ratio of greater than 2.3 during diastole has a sensitivity of 86% and specificity of 95% [6]. The LV systolic function was moderately to severely reduced. There was no thrombus seen and no late gadolinium enhancement was detected (Fig. 1). Coronary angiogram showed normal coronaries. The patient was started on bisoprolol at 5 mg and lisinopril 5 mg. She, however, remained to have palpitation. Repeat ambulatory ECG 10 months later showed PVC burden of 6%. The patient was advised insertion of implantable cardioverter-defibrillator (ICD) but refused.
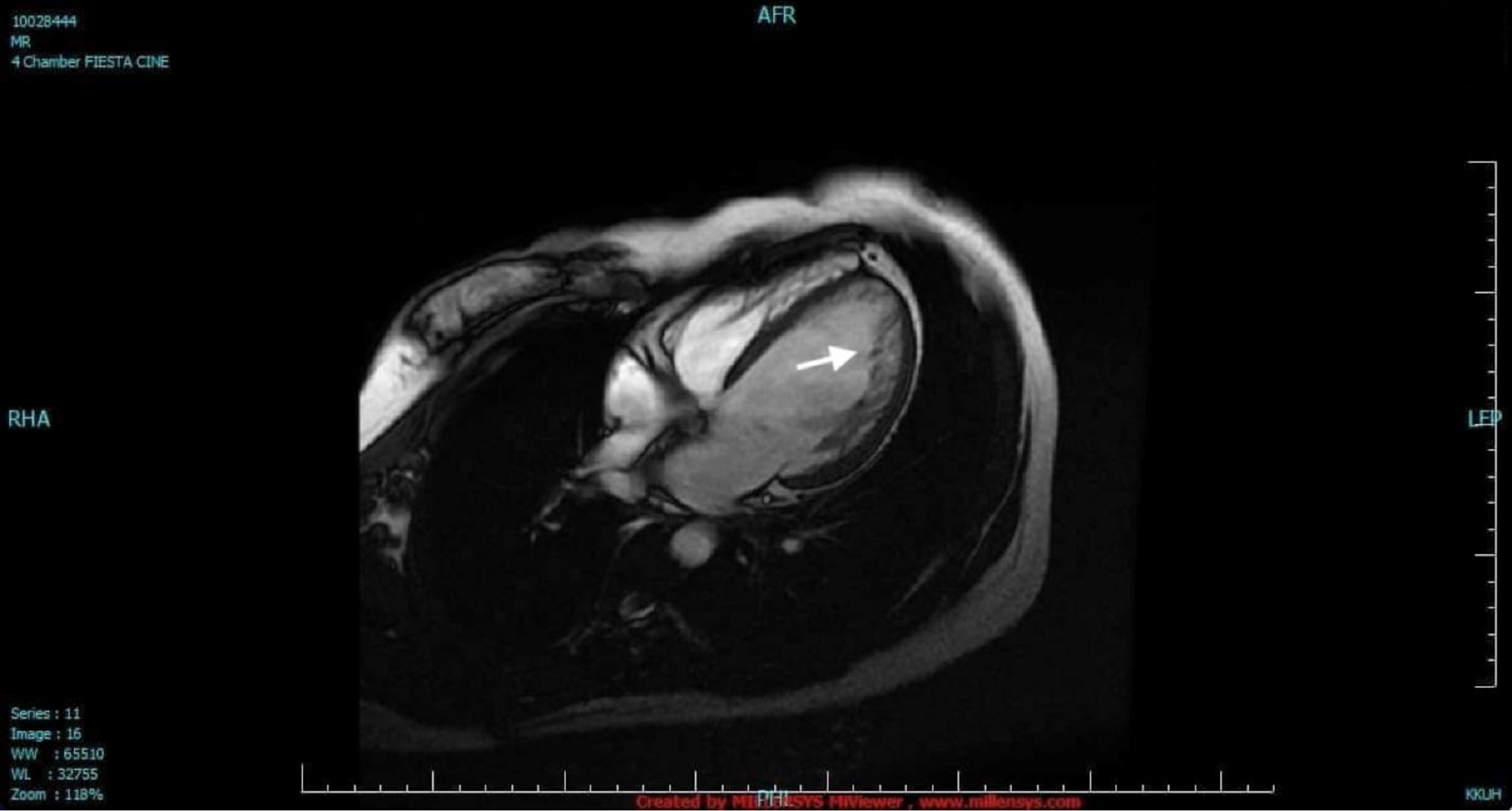 Click for large image | Figure 1. Cardiac magnetic resonance imaging. The LV was dilated with two distinct myocardial layers: a compact layer and a noncompact layer with prominent trabeculations and deep intertrabecular recesses in the lateral wall from base to apex (arrow). The LV systolic function was moderately to severely depressed. LV: left ventricle. |
Upon returning to her native country, the patient consulted a cardiologist and bisoprolol was shifted to amiodarone 200 mg once a day because of persistent episodes of palpitation. Amiodarone relieved the palpitation; however, the patient developed generalized rashes which were attributed to an allergic reaction to the drug. Amiodarone was stopped and bisoprolol 5 mg was resumed. The patient was also started on spironolactone 25 mg.
The patient still remained symptomatic and a year before admission, repeat ambulatory ECG showed PVC burden of 24.5% despite maximum tolerated dose of bisoprolol. Repeat echocardiogram showed improvement of ejection fraction to 48%. Due to the persistence of symptoms and high PVC burden, the patient was again advised to undergo ICD insertion but again refused.
The patient sought alternative option for the frequent PVCs not responsive to the beta-blocker, thus she consulted with the author. There was premature beats on cardiac examination. The rest of the physical examination findings were unremarkable.
Diagnosis
The patient was diagnosed as ventricular arrhythmia (VA), LVNC cardiomyopathy.
Treatment
After thorough discussion of the patient’s condition and the benefits and risks of the procedure, the patient consented for radiofrequency catheter ablation. Beta-blocker was discontinued for 5 half-lives. Baseline ECG showed sinus rhythm, complete left bundle branch block, and monomorphic PVCs in trigeminal pattern (Fig. 2). Endocardial mapping was performed via retrograde transaortic approach. Detailed endocardial electroanatomic mapping (activation and pace-mapping) was performed using a 3.5-mm irrigated-tip catheter (Smarttouch ThermoCool, Biosense Webster Inc., Diamond Bar, CA) and CARTO 3 navigation system. Radiofrequency energy was delivered at the target site for ablation with power settings of up to 40 W, irrigation rate up to 30 mL/min and contact force of 10 - 20 g (Figs. 3 and 4). The PVCs disappeared within 20 s of radiofrequency energy delivery which was continued for 180 s. There was no recurrence of the PVCs in the 30-min monitoring period. The patient became asymptomatic and there was no PVC recorded in telemetry and the patient was discharged the following day. Bisoprolol at 5 mg once daily was resumed for the reduced ejection fraction.
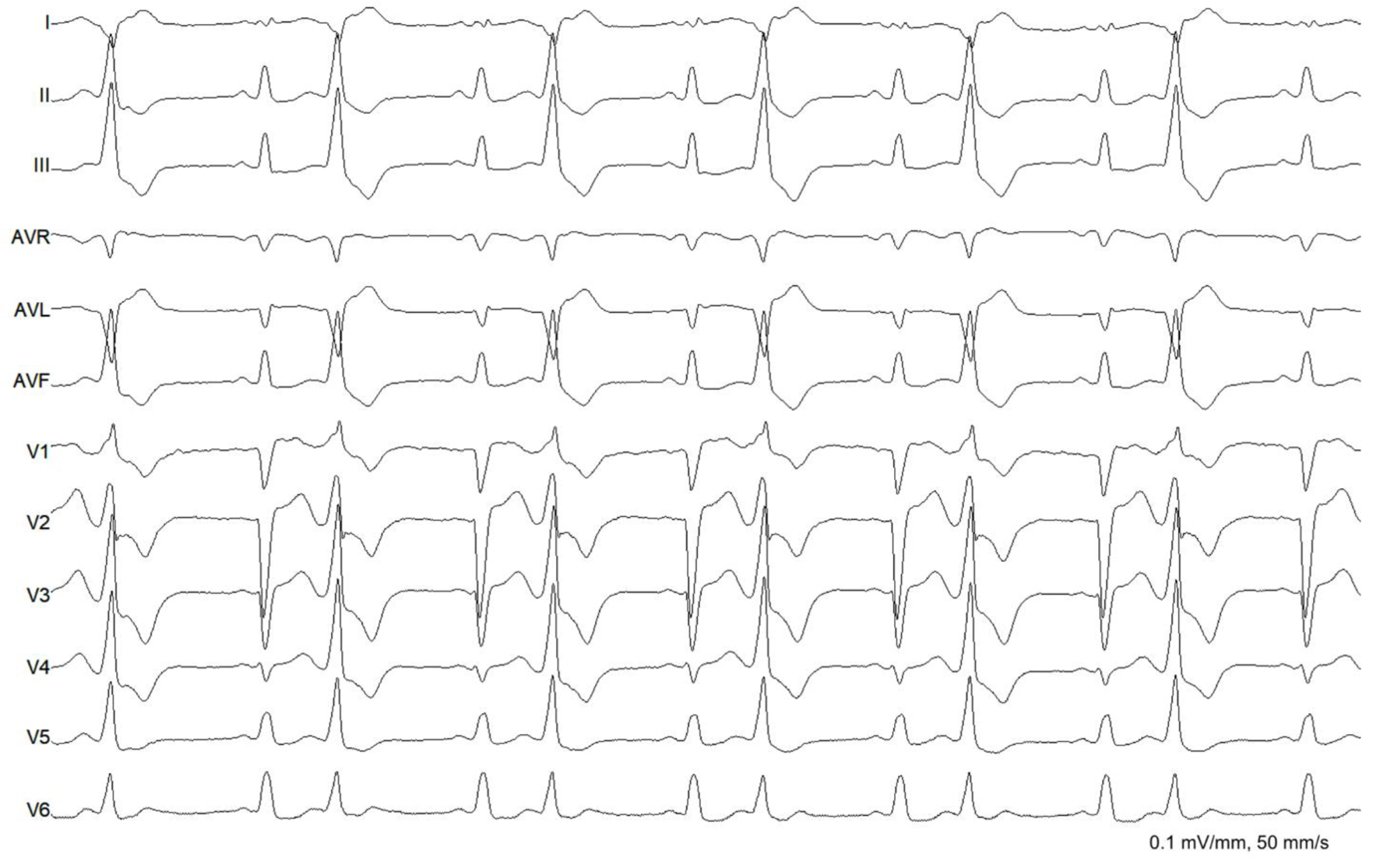 Click for large image | Figure 2. Baseline electrocardiogram. The rhythm was sinus with complete left bundle branch block. There were monomorphic premature ventricular complexes (right bundle branch block morphology, inferior axis, QS in lead I and aVL, QRS duration of 138 ms) in bigeminal pattern. |
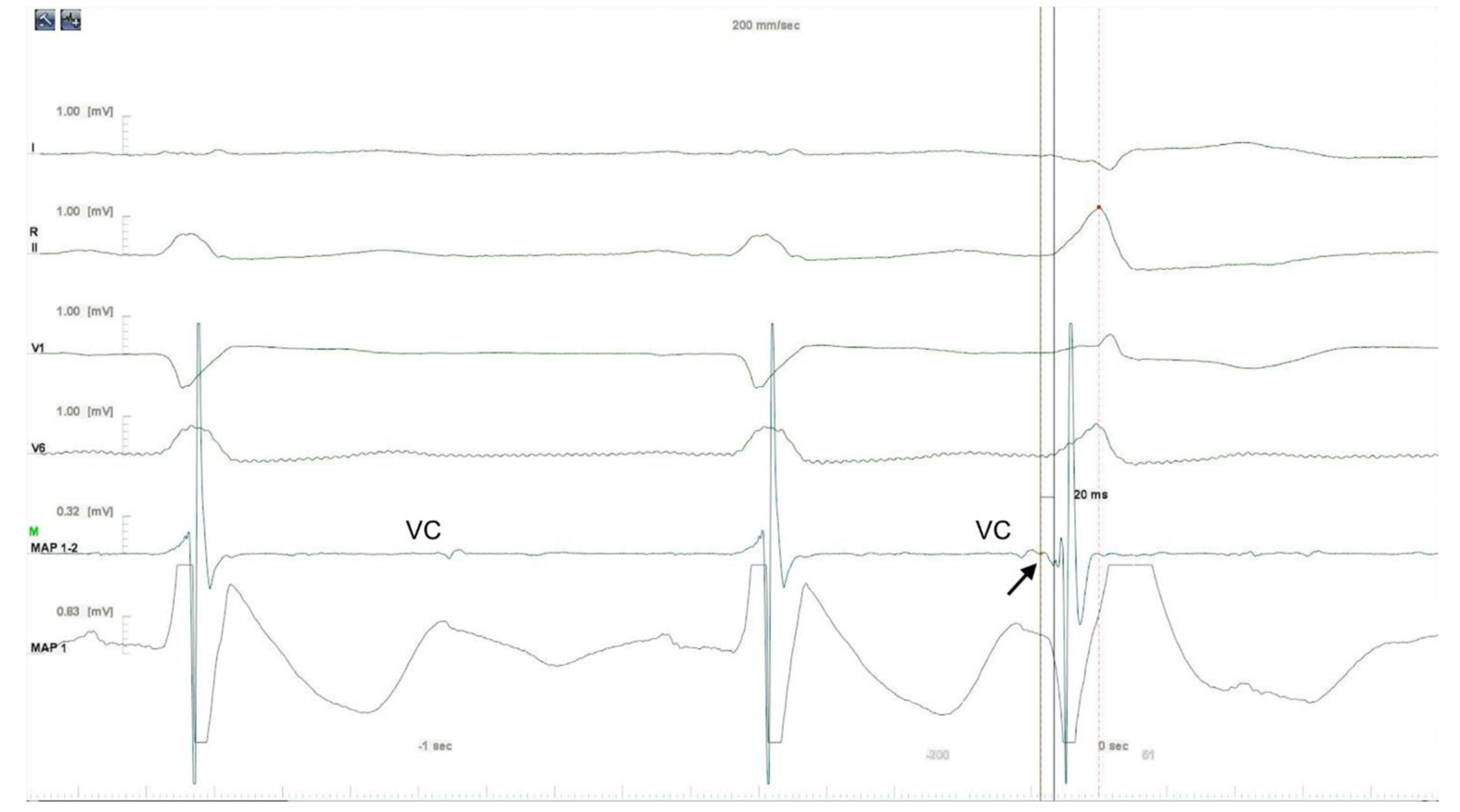 Click for large image | Figure 3. Intracardiac electrogram. The earliest local activation time was recorded in the LV summit. The electrogram in the successful site was preceded by a small presystolic potential by 20 ms (arrow). Aortic valve closure (VC) artifacts were also recorded. LV: left ventricle. |
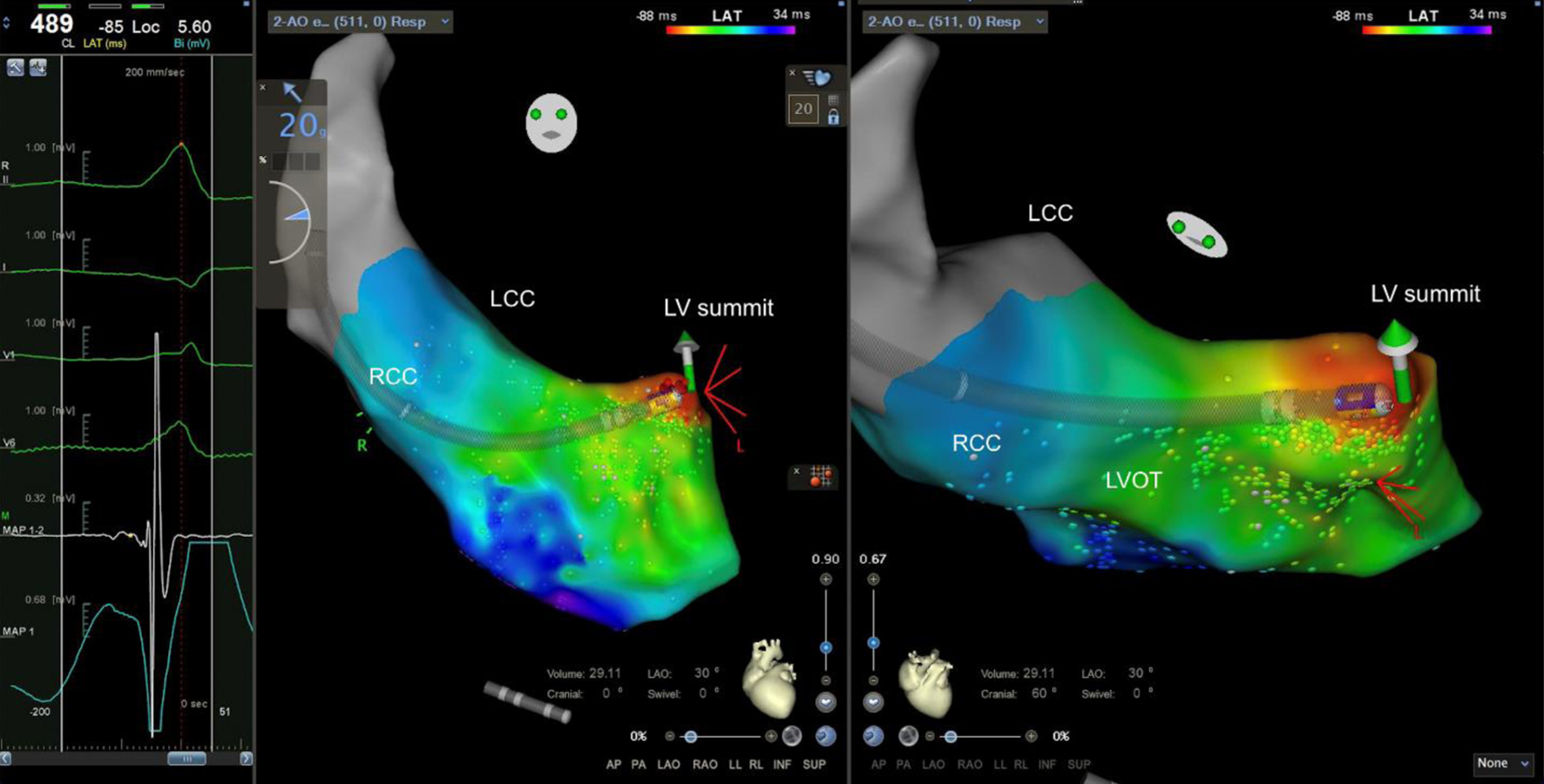 Click for large image | Figure 4. Ablation site (left anterior oblique and cranial views). Ablation was delivered in the site with the earliest local activation time and presence of presystolic potential. RCC: right coronary cusp; LCC: left coronary cusp; LVOT: left ventricular outflow tract; LV: left ventricle. |
Follow-up and outcomes
On follow-up, the patient remained asymptomatic with subjective improvement in effort tolerance. Six months after the radiofrequency catheter ablation, repeat 24-h Holter showed sinus rhythm, complete left bundle branch block with no PVCs. Repeat 2D echocardiogram did not show further improvement in the ejection fraction (LV ejection fraction 46%) and patient was advised to consider angiotensin receptor-neprilysin inhibitor (ARNI) in replacement of the lisinopril and addition of sodium glucose cotransporter 2 (SGLT2) inhibitor.
| Discussion | ▴Top |
VAs are one of the most common complications seen in patients with LVNC. In a series of 34 adult patients with LVNC followed up for a mean duration of 44 months, VAs were seen in 41%, and sudden death in 18%. Other complications were heart failure and thromboembolic events in 53% and 24%, respectively [5]. The VAs in patients with LVNC have been demonstrated to have different origins and mechanisms. In a series of 42 patients with LVNC, three types of VAs were described: those that originated in ventricular outflow tracts, those related to Purkinje potentials, and those that originated in the usual regions of nonischemic cardiomyopathy [7]. In VAs that originated in ventricular outflow tracts (LV outflow tract, left coronary cusp, and LV summit), the presentation of PVCs was more frequent than ventricular tachycardia (VT) and was mostly seen in patients with associated reduced LV systolic function. The pathophysiology of this type of VAs remains unclear given the apparent absence of noncompaction in these areas. One hypothesis is the presence of subendocardial fibrosis not confined to the noncompacted segments in these patients [4]. It can also be postulated that these VAs originating from areas outside of noncompacted areas are independent (e.g., idiopathic VAs) and just coincidental with the presence of isolated LVNC.
The second type was Purkinje-related VA which was defined by mapping or by the presence of Purkinje potentials in regions where ablation was successful. This type was seen most frequently in the group with isolated LVNC. Direct involvement of the fascicular system is postulated to be the origin of these arrhythmias. The third type of VAs that originated from areas of scar with similar patterns to nonischemic cardiomyopathies, predominantly involved the mid inferior septum and basal anterolateral segments. The arrhythmias in these cases could be thought to be associated to the myocardium involved in the noncompaction particularly in the late phases of the disease.
The patient in this case report presented with symptomatic PVCs from the LV summit associated with depressed LV systolic similar to the first group of patients reported in the series by Munoz et al. Cardiac MRI did not show noncompaction or late gadolinium enhancement in the area.
Limited data exist in the medical and interventional therapies for VAs associated with LVNC. Antiarrhythmic drugs, most commonly with beta-blockers, are deemed reasonable. Class III agents, with the exception of amiodarone, are limited by the presence of associated LV dysfunction [8]. ICD implantation is recommended in individuals with LVNC and evidence of ventricular tachyarrhythmias associated with syncope or resuscitated sudden death if meaningful survival greater than 1 year is expected. In patients with an LVNC cardiomyopathy phenotype based on cardiac MRI or echocardiography, implantation of an ICD for primary prevention of sudden cardiac death is considered to follow dilated cardiomyopathy/hypokinetic non-dilated cardiomyopathy (DCM/HNDCM) recommendations and deemed reasonable in individuals with LVNC and evidence of non-sustained VT associated with reduced ejection fraction [9, 10].
Observational studies and isolated reports of patients with drug-refractory VAs have shown that catheter ablation can be performed safely and can achieve long-term arrhythmia-free survival. Nineteen patients in the series of Munoz underwent radiofrequency catheter ablation because of symptomatic VAs and/or impairment of LV function presumably secondary to PVCs/VTs. At the end of the follow-up, eight (42%) patients remained free of arrhythmia and two patients had decrease in PVC burden, four patients had recurrence of VT, one had sudden death, and one required a heart transplant [5]. In another cohort of nine patients with LVNC (eight had LV systolic dysfunction with mean ejection fraction of 40%), patients who presented with frequent PVCs originating from the papillary muscles and/or basal septal regions underwent successful radiofrequency catheter ablation. After median follow-up of 4 years (range 1 - 11) and a mean of 1.8 procedures, VAs recurred only in one patient (11%). Significant improvement in LV function occurred in four of eight cases (50%) [11]. Although there was no further improvement in the LV ejection fraction of this patient 6 months after the radiofrequency catheter ablation, the patient reported improvement in exercise tolerance.
Long-term follow-up is recommended for this patient to monitor recurrence of the VAs either originating from the same site or sites affected by the noncompaction. It is also recommended to monitor the LV systolic function as this has been demonstrated to be a determinant of 5-year survival. In a study by Vaidya et al, reduced LV systolic function and noncompaction extending beyond the apex to the midbasal segments are associated with increased mortality [12]. Thromboprophylaxis is also determined by the LV systolic function [9]. Oral anticoagulation was not considered for the patient because the LV ejection fraction was maintained above 35% at follow-up. Finally, it is also recommended that immediate relatives of the patients are screened for the presence of LVNC [13].
Conclusion
A 37-year-old woman who presented with symptomatic LV summit PVCs and impaired LV systolic function due to LVNC underwent successful radiofrequency catheter ablation. There was no arrhythmia recorded in the intermediate-term follow-up at 6 months. This case further supports the success and safety of radiofrequency catheter ablation in patients with LVNC and VAs. Longer-term follow-up is recommended to monitor LV systolic function and arrhythmia recurrence.
Acknowledgments
None to declare.
Financial Disclosure
There was no funding for this case report.
Conflict of Interest
There is no conflict of interest.
Informed Consent
The author confirms that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Author Contributions
The author performed all the tasks from the conceptualization, creation of the initial draft, review of literature, and revisions.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
Abbreviations
LVNC: left ventricular noncompaction; PVC: premature ventricular complex; LV: left ventricle; MRI: magnetic resonance imaging; VA: ventricular arrhythmia; ICD: implantable cardioverter-defibrillator
| References | ▴Top |
- Filho DCS, do Rego Aquino PL, de Souza Silva G, Fabro CB. Left ventricular noncompaction: new insights into a poorly understood disease. Curr Cardiol Rev. 2021;17(2):209-216.
doi pubmed pmc - Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
doi pubmed - Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44(37):3503-3626.
doi pubmed - Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32(12):1446-1456.
doi pubmed - Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493-500.
doi pubmed - Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46(1):101-105.
doi pubmed - Sanchez Munoz JJ, Munoz-Esparza C, Verdu PP, Sanchez JM, Almagro FG, Ruiz GE, Gimeno Blanes JR, et al. Catheter ablation of ventricular arrhythmias in left ventricular noncompaction cardiomyopathy. Heart Rhythm. 2021;18(4):545-552.
doi pubmed - Sharif ZI, Lubitz SA. Ventricular arrhythmia management in patients with genetic cardiomyopathies. Heart Rhythm O2. 2021;2(6 Part B):819-831.
doi pubmed pmc - Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16(11):e301-e372.
doi pubmed - Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43(40):3997-4126.
doi pubmed - Muser D, Liang JJ, Witschey WR, Pathak RK, Castro S, Magnani S, Zado ES, et al. Ventricular arrhythmias associated with left ventricular noncompaction: Electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2017;14(2):166-175.
doi pubmed - Vaidya VR, Lyle M, Miranda WR, Farwati M, Isath A, Patlolla SH, Hodge DO, et al. Long-term survival of patients with left ventricular noncompaction. J Am Heart Assoc. 2021;10(2):e015563.
doi pubmed pmc - Casas G, Oristrell G, Rodriguez-Palomares J, Borregan M, Limeres J, Silveira I, Gutierrez L, et al. Role of family screening and genetic testing in left ventricular noncompaction. Eur Heart J. 2018;39(Suppl 1):ehy565.P2250.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


