| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 10, October 2022, pages 483-490
Be Patient: Prolonged Extracorporeal Membrane Oxygenation Support Including Full System Switch With Favorable Outcome
Mathias Schmandta, c, Christian Putensena, Tatjana Stiehla, Julia Wagenpfeilb, Jens-Christian Schewea, Stefan Felix Ehrentrauta
aDepartment of Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany
bDepartment of Diagnostic and Interventional Radiology, University Hospital Bonn, Bonn, Germany
cCorresponding Author: Mathias Schmandt, Department of Anesthesiology and Intensive Care Medicine, Venusberg-Campus 1, 53127 Bonn, Germany
Manuscript submitted July 3, 2022, accepted September 21, 2022, published online October 31, 2022
Short title: Prolonged ECMO Support
doi: https://doi.org/10.14740/jmc3979
| Abstract | ▴Top |
Despite tremendous advances in treatment, acute respiratory distress syndrome (ARDS) remains a disease with high mortality (42-48%). Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is often used as a last treatment option, which poses complex problems for the treatment team, especially with prolonged ECMO support. We report an interesting case of a 40-year-old female patient who developed influenza pneumonia leading to ARDS and subsequently requiring ECMO. Due to severe clotting complications, a prolonged ECMO run time with numerous filter changes was required. After a total of 56 days of ECMO therapy, the patient was successfully weaned. Fortunately, further in the course of treatment, complete recovery with restitutio ad integrum was achieved. A distinguishing feature of this case report is the description of a complete ECMO system change and the concurrent use of two ECMO systems for the same patient. Additionally, we provide data on the patient’s current health-related quality of life as measured using the World Health Organization Disability Assessment Schedule 2.0.
Keywords: Viral pneumonia; ARDS; ECMO; WHODAS 2.0
| Introduction | ▴Top |
Acute respiratory distress syndrome (ARDS) is a life-threatening disease, and identifying the underlying cause is crucial for successful treatment [1]. Despite tremendous progress in the treatment of ARDS, the mortality rate (42-48%) remains high [2]. The use of veno-venous extracorporeal membrane oxygenation (VV-ECMO) is a recognized treatment option for severe ARDS, including respiratory failure due to viral pneumonia, such as influenza infection [3]. An analysis of patients with severe ARDS under ECMO therapy conducted by the Extracorporal Life Support Organization in 2009 showed a survival rate of 50% to hospital discharge [4]. Similar results were obtained in the CESAR trial and a Danish study by Lindskov and colleagues [5, 6]. However, both studies assessed relatively short treatment intervals of VV-ECMO support of up to 9 days. Although the benefits of such temporary use of ECMO to bridge the time until sufficient pulmonary recovery are widely accepted, uncertainty remains about the outcomes of patients requiring long-term ECMO therapy [7]. Here prolonged ECMO therapy is defined as a run time > 28 days [8]. Moreover, data from our institutional database collected from over 450 VV-ECMO runs indicate our institution has a median duration of 15 days. Hence, the case described here has a significantly longer ECMO duration compared with both that in the literature and in the regular case distribution at our institution. Apart from the planning and maximum implementation of complex intensive care therapy, the treatment team was also confronted with the ethical question on the decision to continue or withdraw ECMO support, especially if no signs of clinical recovery could be observed despite exhaustive intensive care treatment. We describe the case of a 40-year-old female patient undergoing prolonged ECMO therapy with the rarely reported feature of a complete ECMO circuit change, including the complete exchange of the ECMO cannulas during ongoing therapy.
| Case Report | ▴Top |
Investigations
We describe the rare circumstances of a 40-year-old female patient without preexisting health conditions who developed viral influenza pneumonia leading to ARDS and requiring ECMO.
The initial treatment took place at home. Antibiotic therapy was also started there but was discontinued within less than a week by the family’s general practitioner. The anamnestic information about the preparation used could not be obtained. Due to progressive pulmonary deterioration, the patient was referred to a regional hospital for further treatment. There, the patient had to be transferred to the local intensive care unit (ICU) immediately after admission due to progressive pulmonary dysfunction with severe pyrexia at 40 °C.
As the patient’s clinical condition worsened, calculated therapy with azithromycin, piperacillin/tazobactam, and oseltamivir was started according to the current guidelines [9]. Initially, non-invasive ventilation (NIV) therapy was administered, but it did not provide sufficient stabilization. Hence, the patient underwent intubation. However, since gas exchange was further compromised under lung-protective ventilation, prone positioning was additionally performed, through which the FiO2 requirement improved. Nevertheless, a combination of oxygenation and CO2 elimination disorder developed. Thus, in the absence of improvement, our ARDS/ECMO center was enlisted.
The patient was evaluated at the referring hospital by our ECMO team [10]. Since the patient had stabilized under the initial therapy, the initiation of ECMO therapy could be initially deferred. The patient was transferred to our ARDS/ECMO center for further treatment. Continued lung-protective ventilation resulted in rapid stabilization, along with the de-escalation of ventilation pressures and FiO2 demand.
Diagnosis
The initial computed tomography (CT) scan obtained at our center showed extensive bilateral infiltrates (Fig. 1). In the meantime, the suspected diagnosis of influenza virus pneumonia was confirmed by positive bronchoalveolar lavage via polymerase chain reaction.
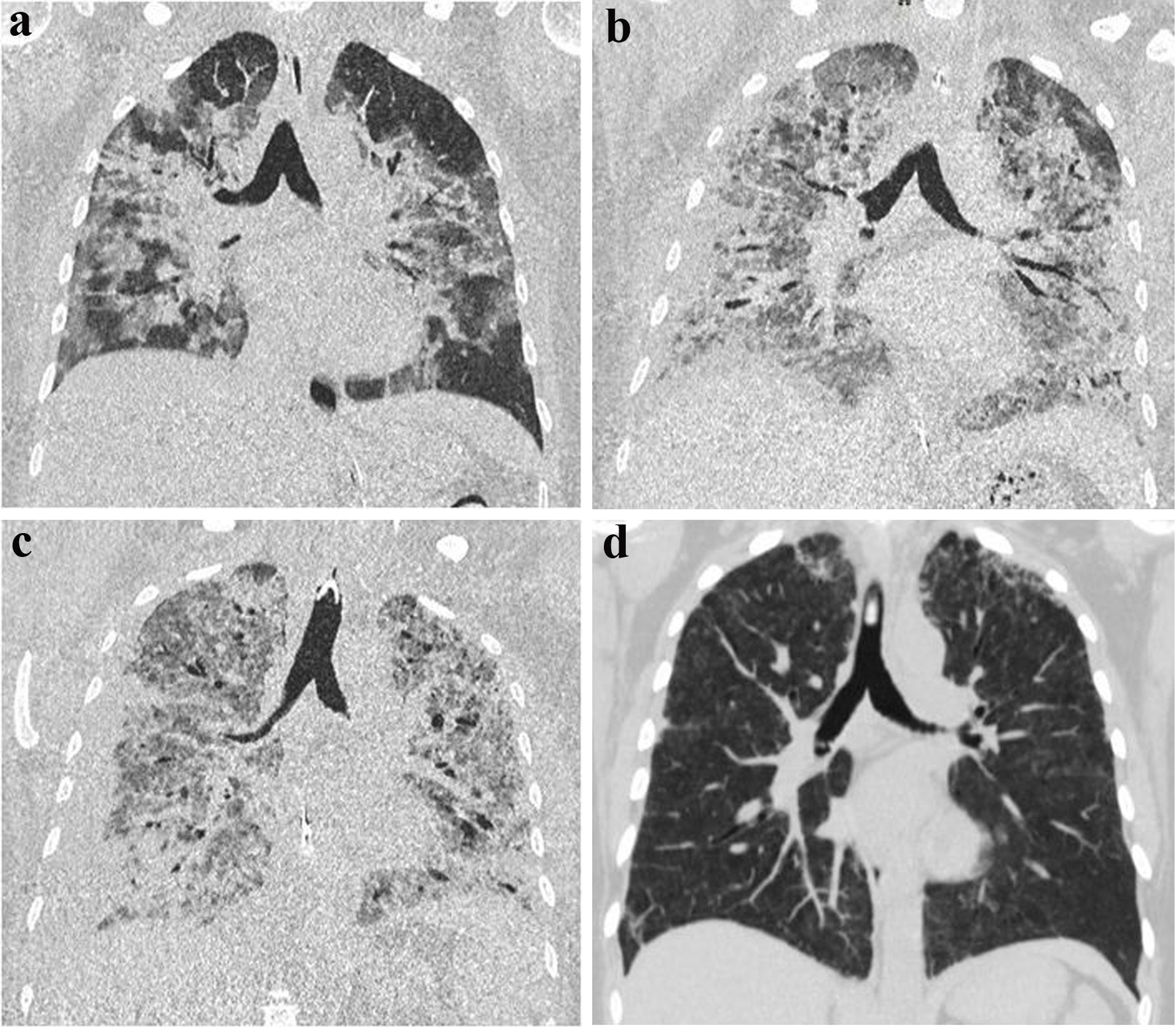 Click for large image | Figure 1. Computed tomography (CT) images during intensive care unit therapy. Chest CT imaging of a 40-year-old patient with marked bilateral influenza pneumonia apically accentuated (a). In the 2-week course, increase of bipulmonary infiltrates with definable consolidations on both sides (b). Another 2 weeks later, further progression of bilateral infiltrates/consolidations in the setting of acute respiratory distress syndrome following influenza pneumonia involving the bilateral lungs and delineable bronchiectasis (c). In the 3-month follow-up, almost complete regression of ubiquitous pneumonic infiltrates with only residual reticular drawing proliferation in the bilateral upper lobes (d). |
Treatment
Despite 7 days of controlled ventilation with biphasic positive airway pressure and full antiviral/antibiotic treatment, the patient underwent a second episode of acute respiratory decompensation with fulminant ARDS with respiratory global insufficiency. This led to the critical reevaluation of ECMO indication and subsequent initiation of veno-venous extracorporeal support based on the recommendations from the EOLIA trial [11].
Even after VV-ECMO implantation, the respiratory situation was still unpromising despite high gas and blood flow. An FiO2 of 0.8 - 1.0 was necessary to achieve a sufficient pO2. Similarly, repeated prone positioning only moderately improved the pulmonary situation.
These developments prompted the decision to establish a third ECMO cannula via the right internal jugular vein to achieve a sufficiently high blood flow, targeting 60-80% of the patient’s cardiac output. Consequently, conditions stabilized, and the treatment continued. The circuit was then established as a bifemoral outlet and jugular inlet configuration.
Given the foreseeable prolonged weaning, a puncture-dilation tracheotomy was performed on day 6. Subsequently, the patient presented with nasopharyngeal bleeding, which could only be temporarily stopped by colleagues in the ear, nose, and throat (ENT) team by tamponade insertion. Therefore, on the following day, renewed hemostasis and surgical widening of the tracheostoma became necessary. Due to several fractured tracheal cartilage braces, the tracheostoma was surgically revised.
Considering alveolitis as the possible cause of ARDS, cortisone was administered at a dose of 250 mg on day 15. Repeat chest CT examinations (Fig. 1) revealed a severely structurally damaged lung with hardly any area for gas exchange remaining.
A co-evaluation with colleagues from the pulmonology department could not identify any further therapeutic options. Given the patient’s seemingly completely destroyed lung, a request for lung transplantation was initiated at the Hanover Medical Clinic at the family’s request. However, this proposition was rejected by the clinic due to the ongoing ECMO therapy.
On day 21, a large pleural effusion (1,700 mL) on the right lung had to be relieved by mini-thoracotomy and a Bulau drainage. Subsequently, on day 23, a persistent, Hb-relevant bleeding from the area of the Bulau drainage incision. CT angiography revealed the bleeding was stemming from a muscle branch of the right thoracic lateral artery, accompanied by bleeding of the fifth intercostal artery. Both vessels were successfully embolized by coil insertion with subsequent cessation of bleeding, as confirmed on follow-up angiography.
Anticoagulation was initially started with heparin IV according to our in-house protocol [12]. With increased bleeding and reduced thrombocytes, heparin-induced thrombocytopenia was suspected and confirmed. This was aggravated by acquired von Willebrand syndrome.
In the presence of this complex coagulation situation, repeat and varying administrations of individual factors, such as factors 8 and 13, were necessary (Table 1).
 Click to view | Table 1. Overview of Observed Bleeding Events in Combination With Administered Blood Products to Control Hemorrhage |
After more than 1 month of therapy, FiO2 was stably maintained at values below 0.5 for the first time. However, the respiratory situation remained unpredictable, and frequent deteriorations occurred. Sufficient oxygenation and carbon dioxide removal remained difficult to achieve. To stabilize the situation, controlled ventilation with the highest possible proportion of spontaneous breathing was required [13]. A complex sedation scheme was implemented to ensure adequate sedation while maintaining spontaneous breathing. An adequate sedation level could only be achieved through four-fold sedation and supplementary therapy with quetiapine and melperone. The patient continued to show obvious signs of agitation. Therefore, the sedation was extended to include the anesthetic conserving device (AnaConDa) system.
On day 25, the pulmonary situation improved slightly, and FiO2 was temporarily reduced to 0.45. On day 31, Hb-relevant bleeding in the tracheostoma area necessitated transfusion and surgical hemostasis. Again, coagulation was optimized by administering coagulation factors (Table 1). The tracheostoma was inconspicuous in the following days.
On day 36 under ECMO therapy, thrombus formation was observed in the ECMO filter and ECMO cannulas. Thus, a new installation of the ECMO cannulas had to be performed in addition to yet another filter system change. Changing ECMO cannulas or ECMO systems is nothing unusual in the context of such a therapy. Due to the situation described above, a complete switch of the ECMO circuit, including ECMO cannulas, became necessary, specifically after several previous filter exchanges due to increased filter pressure (dP). Figure 2 demonstrates the filter and system changes with regard to the therapeutic time course. At this point, the patient still required ECMO support; thus, explantation and reimplantation were unavailable options. Consequently, we had to establish a virgin secondary ECMO system while the initial ECMO system remained.
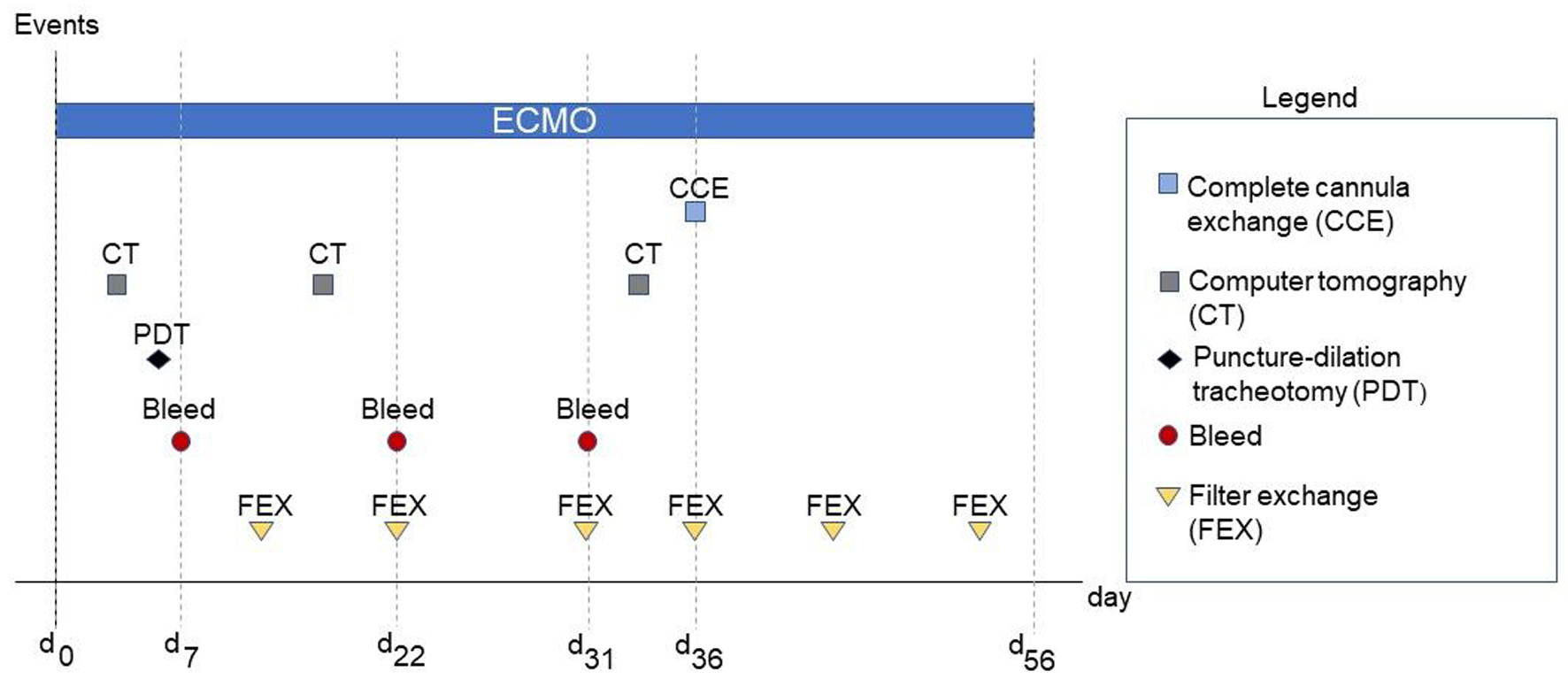 Click for large image | Figure 2. Relevant events during the treatment period. dx refers to the respective day following extracorporeal membrane oxygenation (ECMO) implantation d0. |
Accordingly, one outlet cannula was first removed from the left femoral vein. ECMO therapy was maintained by overlapping the remaining outlet cannula in the right femoral vein and the inlet cannula in the right internal jugular vein. Next, the left internal jugular vein was cannulated as an inlet and the left femoral vein as an outlet. The second ECMO was thus finally established. Thus, two ECMO devices were temporarily in simultaneous use. After successful initiation of the second circuit, the first ECMO device was removed without complications. With a sufficient blood flow and sufficient gas exchange under an FiO2 of 0.4, the installation of a third ECMO cannula was not necessary (Fig. 3).
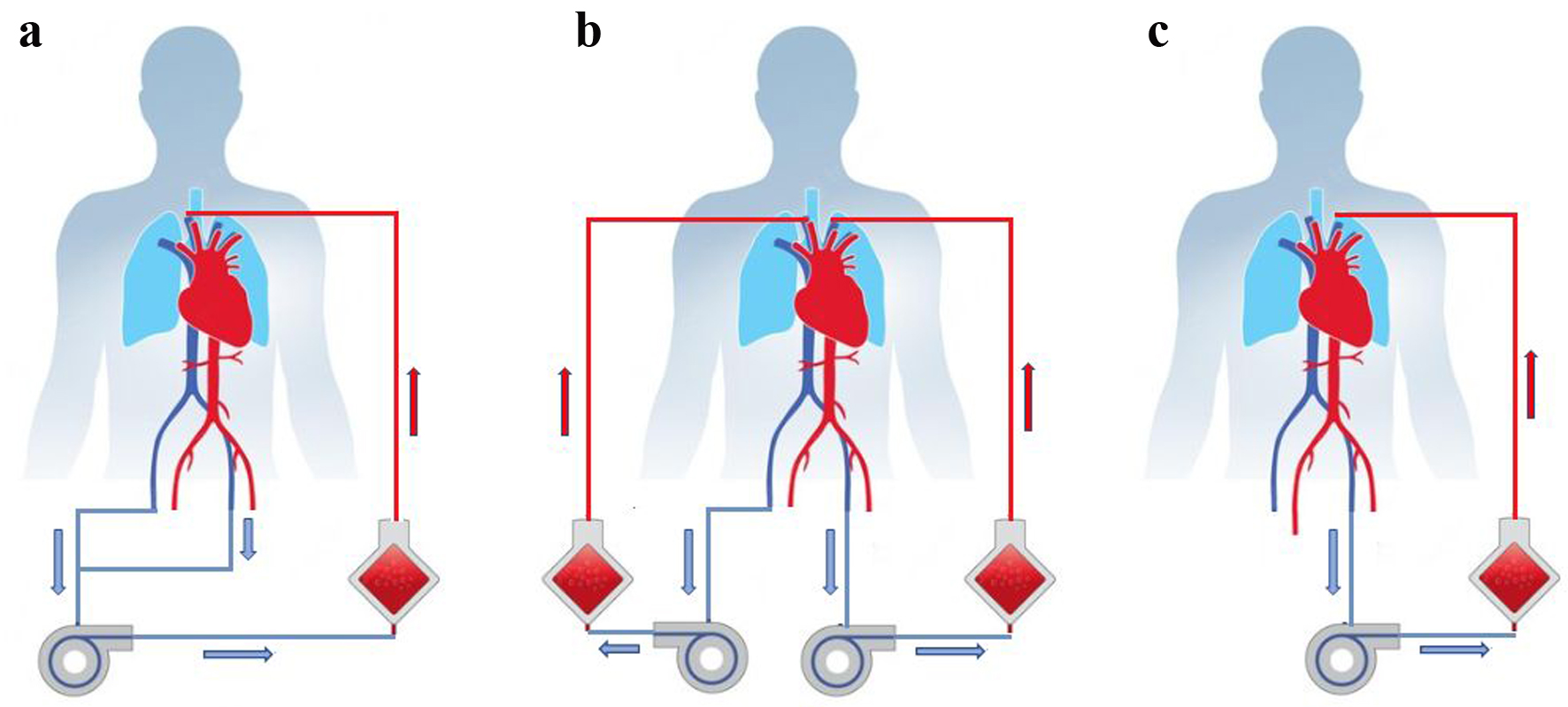 Click for large image | Figure 3. (a) Initial extracorporeal membrane oxygenation (ECMO). (b) Temporal use of two ECMO devices at the same time. (c) ECMO circuit after complete exchange. |
Clinically, however, increasing improvement of the pulmonary situation could be observed. Thus, pulmonary weaning was started on day 41. With simultaneously reduced sedation, sufficient ventilation could be established under airway pressure release ventilation (APRV). A strict protocol with a slow increase of the continuous positive airway pressure (CPAP) phases was established.
Thus, a timed trial was first performed for approximately 20 min under CPAP/assisted spontaneous breathing (ASB). The patient showed calm spontaneous breathing and stable gas exchange. Subsequently, the CPAP/ASB phases were continuously extended over a further 5 days to up to 30 min per shift. After this was achieved, the next step was to start ECMO weaning. Blood flow was reduced to 2 L/min and gas flow to 0.5 L/min so that ECMO therapy could be terminated on day 56 when respiratory conditions became stable.
After the end of ECMO therapy, further weaning from mechanical ventilation was continued, as follows: CPAP mode for 120 min, then at least 2 h break. After the CPAP interval, breathing at the “T-piece” followed for 5 - 10 min with 4 L O2/min. Outside the weaning phases, the patient was ventilated in biphasic positive airway pressure mode.
Further into the course of treatment, the time without mechanical pressure support on the T-piece could be extended stepwise. Due to ICU-acquired critical muscle weakness, a speech cannula could not yet be attached due to a high risk of aspiration.
A flexible endoscopic evaluation of swallowing performed on day 80 showed no abnormalities. Consequently, first attempts with a speech cannula were made with good success, and the use was eventually extended.
Complete weaning from the respirator was finally achieved at day 107 after initial intubation.
Neurologically, a complete remission was noted. The patient received regular physiotherapeutic care, resulting in full mobility at the time of hospital discharge.
The patient was successfully decannulated after reevaluation by the ENT colleagues and then successfully discharged.
Follow-up and outcomes
We performed regular follow-up by telephone with the patient. To assess functional wellbeing after full recovery, we performed the World Health Organization Disability Assessment Schedule 2.0, 36-item version [14]. To determine any negative impact on health-related quality of life (HrQoL), the patient was asked to perform a self-assessment of her status prior to ECMO and 12 months after discharge. We chose a period of > 12 months to allow for full recovery and for the patient to perform the test in a steady state. At this point, any observed changes were most likely permanent. An overall score of 18.68% indicated a high level of disability, putting the patient into the 80th percentile compared with the population distribution (Fig. 3, left panel). The “pre-ECMO” assessment revealed no discernible disabilities in any of the tested domains (participation in society; life activities; getting along with people; self-care; getting around; and understanding and communicating) (Fig. 3, right panel). Analysis of the “post-ECMO” situation revealed a marked increase in four out of six test domains (getting around; understanding and communicating; participation in society; and life activities). While the patient reflected her own situation positively and stated that she saw no direct impairment in her HrQoL, the high scores show the lasting impact ECMO treatment and the associated ICU stay have on HrQoL.
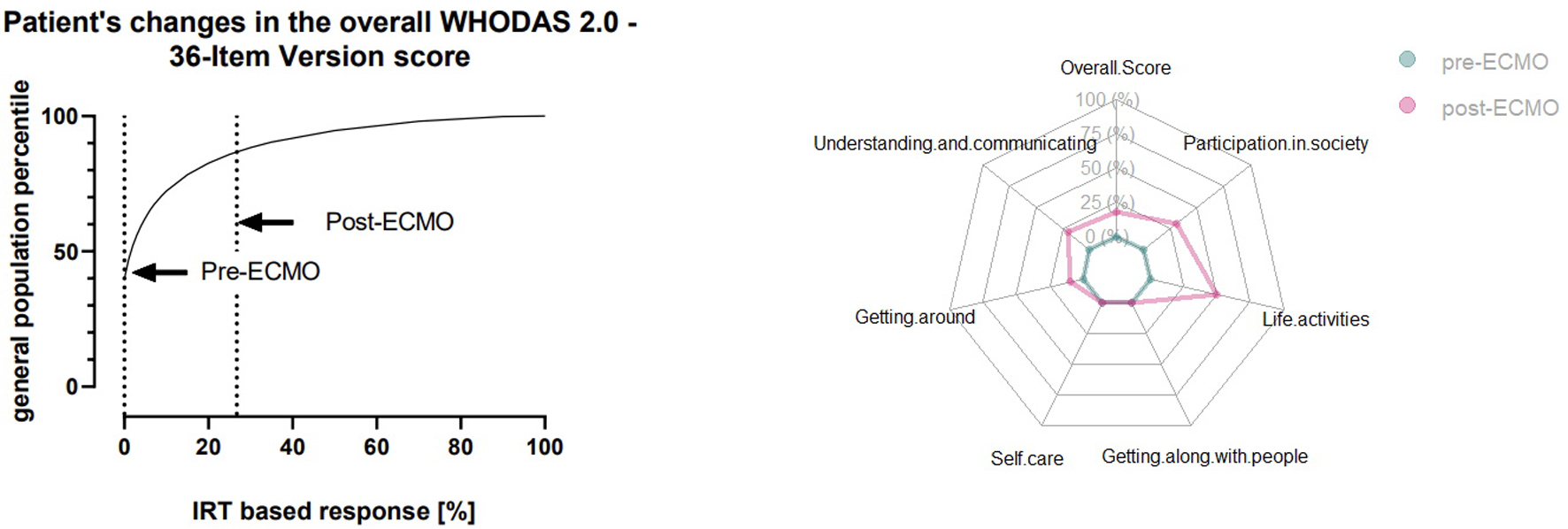 Click for large image | Figure 4. Patient’s World Health Organization Disability Schedule 2.0 (WHODAS 2.0) score assessed by self-reporting. Left panel: patient’s WHODAS 2.0 overall score before and after extracorporeal membrane oxygenation (ECMO; dashed lines) compared with the general population score. Right panel: domain-specific score before and after ECMO. |
| Discussion | ▴Top |
ARDS impairs the gas exchange and in severe cases can lead to a refractory respiratory global insufficiency with effects on the pH [15]. This situation can be bridged by the use of ECMO as a well-established treatment. The initiation should be by early, no less than 7 days after the start of invasive mechanical ventilation to prevent ventilation-associated lung injury, which stems in part from excessive tidal volumes of > 6 mg/kg body weight [6, 16, 17].
However, no studies or guidelines have specified the duration of the life-sustaining extracorporeal procedure. Serious and protracted ECMO therapies in expectation of a cure should always be balanced by the medically necessary prudence, which also allows for a timely termination of therapy in the absence of response. For this purpose, family members should be informed about the therapeutic limits of ECMO therapy at an early stage [18].
The start of ECMO therapy in the case presented here was on day 7 after the initiation of invasive ventilation with a mean predicted survival of 75% by the respiratory ECMO survival prediction (RESP) score [19].
The RESP score cannot be used to make any statement about possible in-hospital progression. In our case, we had to assume an underscoring and thus an overestimation of the internal survival probability, since clinical complications, such as intrapulmonary hemorrhage, which prolong the therapeutic course cannot be predicted.
Despite > 14 days of ECMO support without improvement and negative evaluation for lung transplantation, the patient continued to have single-organ failure. Thus, even after considering all the findings, a limitation of therapy was not a justifiable option.
The ethical burden of making a decision to continue ECMO therapy is difficult to bear, especially when no signs of improvement could be observed despite exhaustive intensive care causal and supportive therapy.
Since in this case only single-organ failure occurred and the patient was young with no previous illnesses, the therapy was continued despite no significant improvement in the pulmonary situation for weeks. The key to the ultimate success in this case was the successful management of rare complications, and, most importantly, the concomitant use of two ECMO circuits for an individual patient. Such a procedure has not been reported in other similar case reports, as well as in the current literature. Previously reported cases of hybrid/dual ECMO cases have involved patients with hyperdynamic sepsis where a secondary circuit was necessary to achieve sufficient oxygenation.
In identifying possible positive predictors for this ultimately successful course of therapy, the following aspects can also be mentioned in comparison with other case reports and studies: 1) The therapy with ECMO was started within 7 days to allow for lung-protective ventilation [16, 20]. 2) The patient was tracheotomized on day 6 after the start of ECMO therapy. This allowed the patient to be weaned while still under ECMO from the time of clinical stabilization. Thus, the capacity of the respiratory musculature could be increased in direct temporal correlation. The ideal timing of tracheostomy in critically ill patients remains controversial. Young et al [21] found no significant correlation between in-house mortality in 909 patients undergoing surgery and tracheostomy before postoperative day 4 or after day 10. This observation apparently holds true in patients who underwent ECMO support, whether VV or veno-arterial, as indicated by the findings of Salna et al [22]. They concluded that tracheostomy can be safely performed during ECMO therapy. Notably, the authors believe that the maintenance or brief cessation of anticoagulation is not associated with a higher incidence of thromboembolism [23]. 3) Our PTT target of 35 s was chosen to minimize the risk of possible lethal complications, including but not limited to intracerebral hemorrhage, which was observed in a recent study by Seeliger et al [23]. In their comparison of high versus low heparinization under ECMO, the high-heparin group had seven cases of intracranial bleeding. Conversely, the low-heparin cohort from our center had no cases of intracranial bleeding. However, this is at the cost of more frequent, clotting-related ECMO system changes. In the case presented here, our lower heparinization approach has not positively impacted the bleeding complications that developed in this case. However, with clinically relevant bleeding complications, an extended target range for activated partial thromboplastin time to minimize system changes was not feasible. 4) A complete change of both the initial ECMO cannulas and the ECMO circuit, made necessary by cannula clotting, can be achieved by the concurrent implantation of a second circuit, as described earlier.
Despite the complicated disease course, the patient was successfully weaned from ECMO after 56 days.
Learning points
As learning points, as well as in comparison with other previously published reports, the following can be noted: if explantation and reimplantation is not an option because the patient continues to require ECMO support, the short-term overlapping use of two ECMO systems described in this report may be a reasonable option.
Conclusion
The decision to continue ECMO therapy should not be made solely on the basis of the duration of therapy already completed. As long as no more than one organ system is affected by organ failure (e.g., the lungs), no signs of pulmonary fibrosis on radiologic imaging are noted, and spontaneous complete recovery is considered possible, maximum therapy should be continued. Moreover, this case demonstrates full recovery without significant impairment of HrQoL.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have no conflict of interest to declare.
Informed Consent
Not applicable due to complete anonymization of patient data.
Author Contributions
Mathias Schmandt: manuscript preparation. Christian Putensen: critical revision. Tatjana Stiehl: data acquisition and evaluation according to World Health Organization Disability Assessment Schedule 2.0, 36-item version. Julia Wagenpfeil: image processing and description. Jens-Christian Schewe: critical revision. Stefan Felix Ehrentraut: manuscript preparation and project supervision.
Data Availability
Any inquiries regarding the availability of the data used and/or obtained in this study should be directed to the corresponding author.
Abbreviations
ARDS: acute respiratory distress syndrome; aPTT: activated partial thromboplastin time; APRV: airway pressure release ventilation; AnaConDa: anesthetic conserving device; BIPAP: biphasic positive airway pressure; BAL: Bronchoalveolar lavage; pCO2: carbon dioxide partial pressure; CPAP/ASB: continuous positive airway pressure and assisted spontaneous breathing; CT: computed tomography; FEES: flexible endoscopic evaluation of swallowing; ELSO: Extracorporeal Life Support Organization; ECMO: extracorporeal membrane oxygenation; HrQoL: health-related quality of life; ICU: intensive care unit; FiO2: fraction of inspired oxygen; NIV: non-invasive ventilation; PO2: partial oxygen pressure; RESP: respiratory ECMO survival prediction
| References | ▴Top |
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562-572.
doi pubmed - ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533.
doi - Zangrillo A, Biondi-Zoccai G, Landoni G, Frati G, Patroniti N, Pesenti A, Pappalardo F. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17(1):R30.
doi pubmed - Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35(12):2105-2114.
doi pubmed - Lindskov C, Jensen RH, Sprogoe P, Klaaborg KE, Kirkegaard H, Severinsen IK, Lorentsen AG, et al. Extracorporeal membrane oxygenation in adult patients with severe acute respiratory failure. Acta Anaesthesiol Scand. 2013;57(3):303-311.
doi pubmed - Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-1363.
doi - Kon ZN, Dahi S, Evans CF, Byrnes KA, Bittle GJ, Wehman B, Rector RP, et al. Long-term venovenous extracorporeal membrane oxygenation support for acute respiratory distress syndrome. Ann Thorac Surg. 2015;100(6):2059-2063.
doi pubmed - Lepper PM, Barrett NA, Swol J, Lorusso R, Di Nardo M, Belliato M, Belohlavek J, et al. Perception of prolonged extracorporeal membrane oxygenation in Europe: an EuroELSO survey. Perfusion. 2020;35(1_suppl):81-85.
doi pubmed - Ewig S, Hoffken G, Kern W, Rohde G, Flick H, Krause R, et al. Behandlung von Erwachsenen Patienten mit ambulant erworbener Pneumonie und Pravention - Update 2016. Pneumologie. 2016;70(3):151-200.
doi pubmed - Ehrentraut SF, Schroll B, Lenkeit S, Ehrentraut H, Bode C, Kreyer S, Kogl F, et al. Interprofessional two-man team approach for interhospital transport of ARDS-patients under extracorporeal membrane oxygenation: a 10 years retrospective observational cohort study. BMC Anesthesiol. 2019;19(1):19.
doi pubmed - Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965-1975.
doi pubmed - Kreyer S, Muders T, Theuerkauf N, Spitzhuttl J, Schellhaas T, Schewe JC, Guenther U, et al. Hemorrhage under veno-venous extracorporeal membrane oxygenation in acute respiratory distress syndrome patients: a retrospective data analysis. J Thorac Dis. 2017;9(12):5017-5029.
doi pubmed - Wrigge H, Zinserling J, Neumann P, Muders T, Magnusson A, Putensen C, Hedenstierna G. Spontaneous breathing with airway pressure release ventilation favors ventilation in dependent lung regions and counters cyclic alveolar collapse in oleic-acid-induced lung injury: a randomized controlled computed tomography trial. Crit Care. 2005;9(6):R780-789.
doi pubmed - Ustun TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, Saxena S, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ. 2010;88(11):815-823.
doi pubmed - Zampieri FG, Mendes PV, Ranzani OT, Taniguchi LU, Pontes Azevedo LC, Vieira Costa EL, Park M. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care. 2013;28(6):998-1005.
doi pubmed - Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347-354.
doi pubmed - Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226(4):544-564; discussion 565-546.
doi pubmed - Khan R, Anandamurthy B, McCurry K, Krishnan S. Utility of extracorporeal membrane oxygenation in COVID-19. Cleve Clin J Med. 2020.
doi pubmed - Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, Scheinkestel C, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189(11):1374-1382.
doi pubmed - Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
doi pubmed - Young D, Harrison DA, Cuthbertson BH, Rowan K, TracMan C. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121-2129.
doi pubmed - Salna M, Tipograf Y, Liou P, Chicotka S, Biscotti M, 3rd, Agerstrand C, Abrams D, et al. Tracheostomy is safe during extracorporeal membrane oxygenation support. ASAIO J. 2020;66(6):652-656.
doi pubmed - Seeliger B, Dobler M, Friedrich R, Stahl K, Kuhn C, Bauersachs J, Steinhagen F, et al. Comparison of anticoagulation strategies for veno-venous ECMO support in acute respiratory failure. Crit Care. 2021;24(1):701.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


