| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 8, August 2022, pages 421-426
Spontaneous Acute Mesenteric Thrombosis in a Patient With Hemoglobin E Thalassemia
Joseph Asemotaa, Ademola S. Ojoa, d, Somtochukwu G. Ojukwua, Mohammed Salehb, Ravi Sarmac
aDepartment of Internal Medicine, Howard University Hospital, Washington, DC, USA
bDepartment of Medicine, University of Missouri, Columbia, MO, USA
cDepartment of Medicine, Hematology/Oncology Division, Howard University Hospital, Washington, DC, USA
dCorresponding Author: Ademola S. Ojo, Department of Internal Medicine, Howard University Hospital, Washington, DC, USA
Manuscript submitted June 14, 2022, accepted July 29, 2022, published online August 19, 2022
Short title: Acute MVT in a Patient With HbE Thalassemia
doi: https://doi.org/10.14740/jmc3969
| Abstract | ▴Top |
Acute mesenteric vein thrombosis (MVT) is an uncommon cause of mesenteric ischemia and portal hypertension. The common risk factors for the development of MVT include surgery, acute-intraabdominal inflammatory disorders, malignancies, and other prothrombotic states. However, MVT can occur in the absence of these risk factors. We describe the case of a 40-year-old man with a new diagnosis of hemoglobin E thalassemia and MVT and discuss the relationship between the hemoglobinopathy and thrombosis based on evidence from existing literature. The case emphasizes the importance of a high index of suspicion in the diagnosis of MVT in other to reduce complications and improve overall outcomes.
Keywords: Mesenteric thrombosis; Mesenteric vein; Hemoglobinopathies; Hemoglobin E; Thalassemia
| Introduction | ▴Top |
Acute mesenteric vein thrombosis (MVT) with or without the involvement of the portal vein is an uncommon cause of mesenteric ischemia and portal hypertension with an estimated incidence of 2.7 in 100,000 [1]. It accounts for an estimated 1 in 5,000 to 15,000 inpatient admissions and 1 in 1,000 emergency surgical laparotomies for acute abdomen annually [2]. MVT is slightly more common in males than females, with an average age-at-diagnosis of 45 to 60 years [3]. The risk of thrombosis is generally low before the fourth decade of life, with a steep increase beginning in the fifth decade [4]. The increase in risk is thought to be due to a rise in comorbidities that potentiate Virchow’s triad of stasis, hypercoagulability, and endothelial dysfunction [5].
Although the common risk factors for the development of MVT include surgery, acute-intraabdominal inflammatory disorders, malignancies, and other prothrombotic states, emerging evidence has shown an increased risk of thromboembolic events including MVT in some hemoglobinopathies [3, 6]. Herein, we describe the case of a 40-year-old man with hemoglobin E (HbE) thalassemia. The case emphasizes the importance of early diagnosis and management in preventing complications and improving overall outcomes. We also provide a detailed review of the existing literature.
| Case Report | ▴Top |
Investigations
A 40-year-old man with a history of alcohol use and stomach ulcer presented to the emergency department because of abdominal pain of 2 weeks duration. The pain was epigastric in location and said to have started gradually. It was described as colicky, intermittent, and rated as 9/10 intensity at its peak. He reported partial relief from the use of antacids. There was a positive history of associated constipation that began 2 days before presentation, for which he used over-the-counter laxatives. He however subsequently developed diarrhea and had about eight episodes of non-bloody loose stool. He reported a loss of appetite and generalized fatigue. The review of other systems was negative. He denied the use of any routinely prescribed medication but admitted to active marijuana use. He had no known food or drug allergies. Physical examination revealed a soft abdomen with tenderness in the epigastric region. There was no guarding or rebound tenderness and bowel sounds were normoactive. Other physical findings were unremarkable.
Diagnosis
Complete blood count revealed hemoglobin of 13.1 g/dL, hematocrit of 42.9%, and mean corpuscular volume of 63 fL. Hemoglobin analysis revealed HbE with thalassemia (a new diagnosis for the patient). Contrast-enhanced computed tomography scan of the abdomen was done and it showed thrombosis of the superior mesenteric vein (SMV) with involvement of splenic and portal veins (Figs. 1 and 2). There was a complete obstruction of the SMV and a near-total occlusion of the portal vein. Due to the absence of clear risk factors for thrombosis, the patient was extensively evaluated for hypercoagulability. This includes alpha-fetoprotein, factor II mutation, factor V Leiden, Jak2mut, paroxysmal nocturnal hemoglobinuria, hepatitis B and C panels, carbohydrate antigen 19-9, intrinsic factor, and beta-2 glycoprotein antibody, all of which were negative.
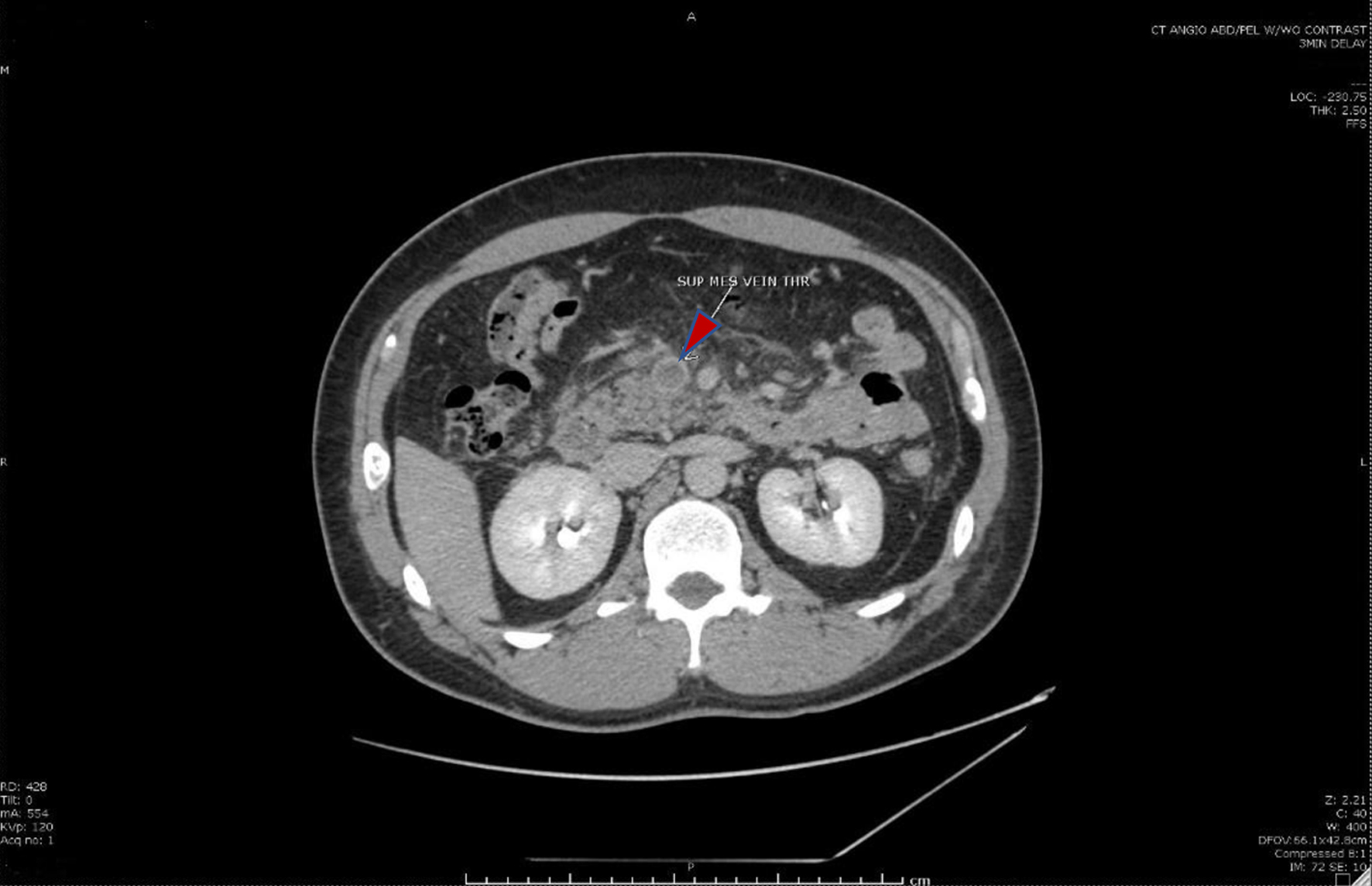 Click for large image | Figure 1. Cross-sectional computed tomography image of the abdomen showing the superior mesenteric vein (red arrow) with intraluminal thrombus. |
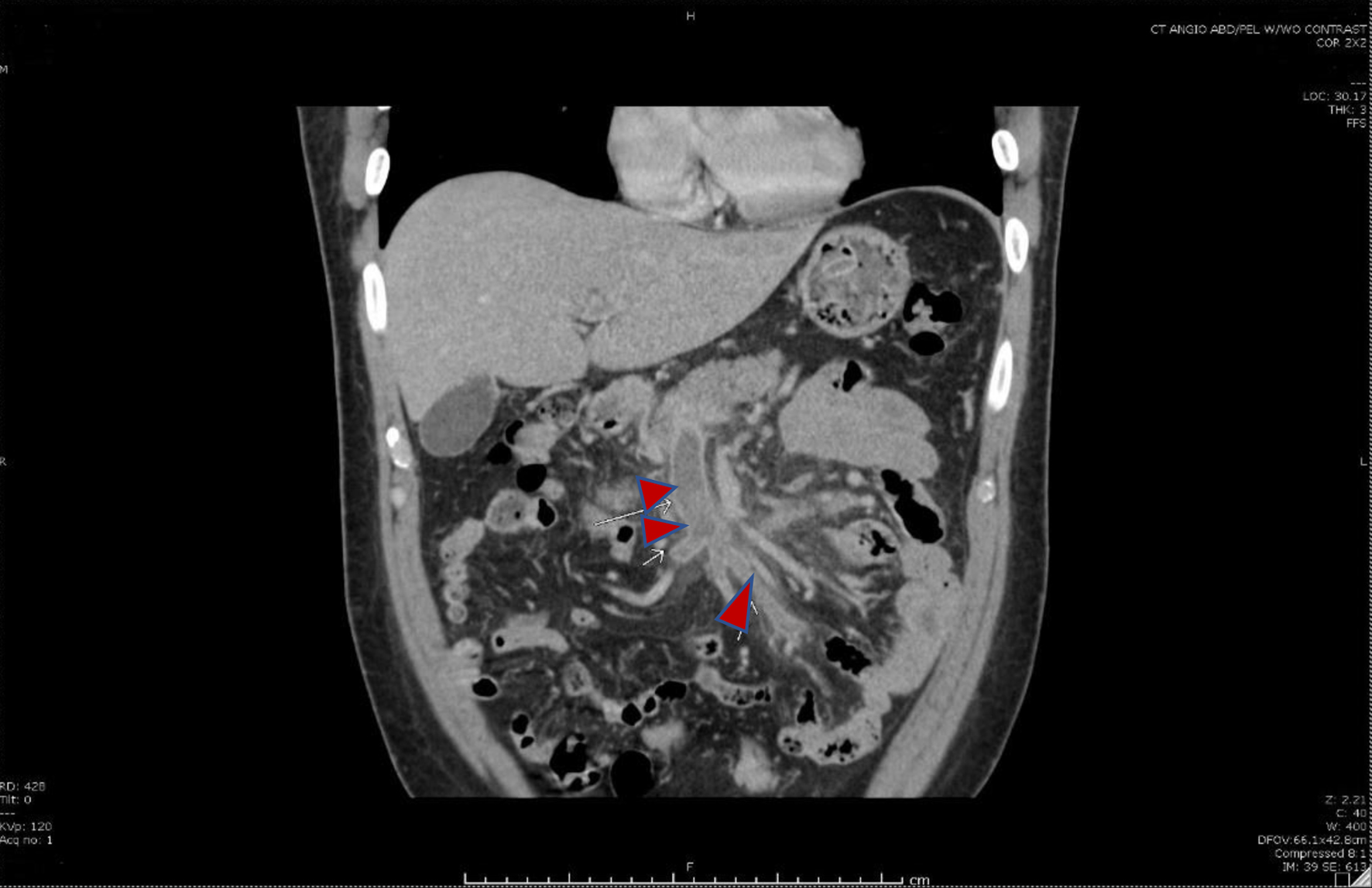 Click for large image | Figure 2. Coronal image showing thrombosis of the superior mesenteric vein and its tributaries (red arrows). |
Treatment
He was commenced on therapeutic low molecular weight heparin at a dose of 80 mg every 12 h, proton pump inhibitors, pain medication, and placed on nil per os (nothing by mouth). The gastroenterology and hematology/oncology services were consulted for expert guidance in management. Aside from anticoagulation, additional interventions such as thrombolysis or thrombectomy were not recommended.
Follow-up and outcomes
Following abatement of the patient’s acute symptoms, he was discharged home on anticoagulation with eliquis, proton pump inhibitors, and instructions to follow up with the gastroenterology and oncology outpatient clinics. At the time of his discharge, no other etiology of his SMV thrombosis was found. Repeat imaging obtained during follow-up (6 months after the initial imaging study) demonstrated features of persistent occlusion and chronic MVT with the development of collateral circulation (Fig. 3). His anticoagulation was continued for another 3 months until his next visit.
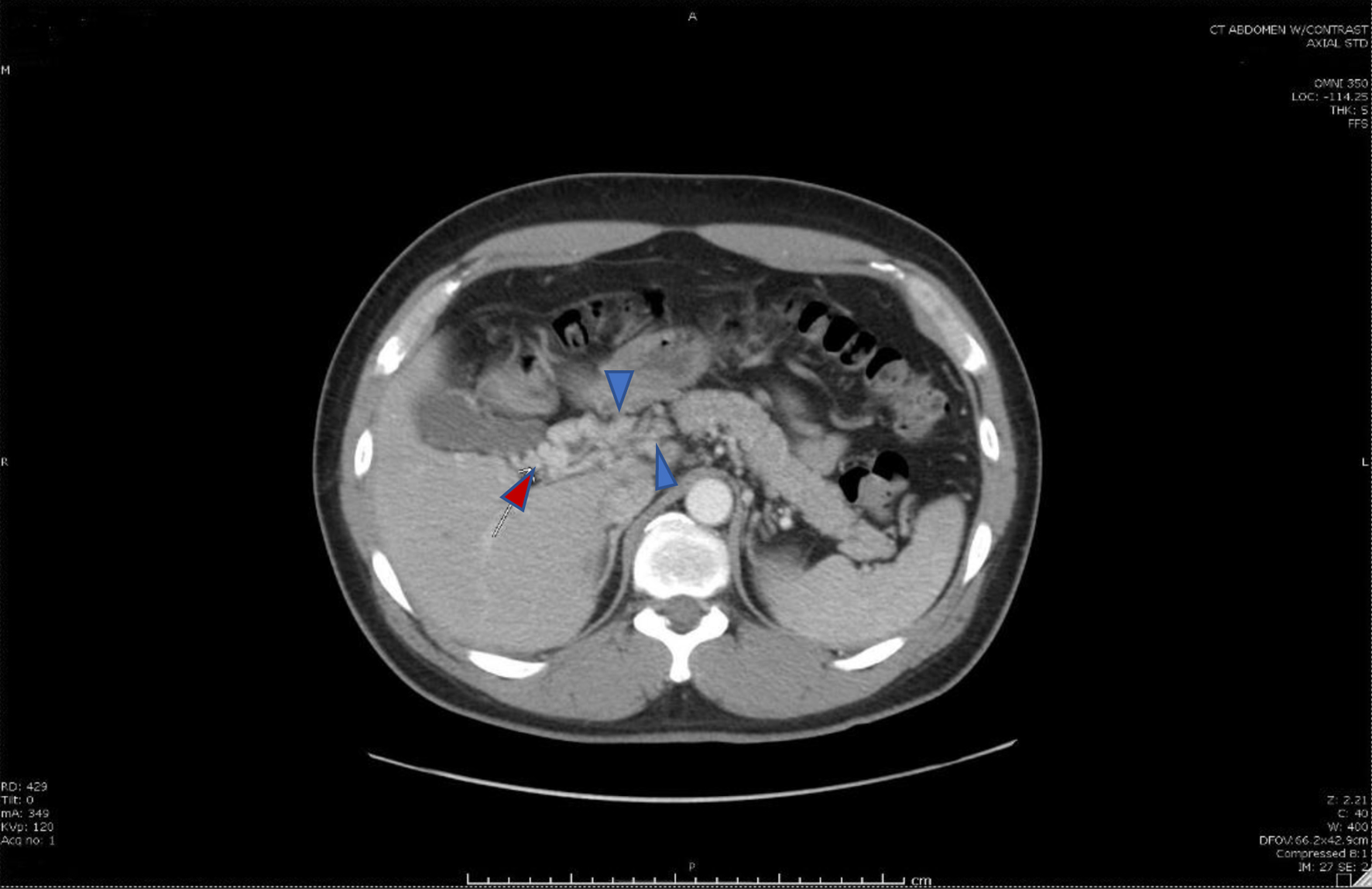 Click for large image | Figure 3. Cavernous transformation of the portal vein (red arrow), a sequela of portal vein thrombosis with an interval development of multiple venous collaterals (blue arrows) in the mesenteric fat, porta hepatis, and adjacent to the head of the pancreas secondary to the chronic portal vein and superior mesenteric vein thromboses. |
| Discussion | ▴Top |
HbE is a common hemoglobin variant in many Asian populations where thalassemias are equally common [7]. It is a β-hemoglobin abnormality resulting from a replacement of lysine with glutamic acid on position 26 of the β-chain leading to a reduction in the β-chain synthesis [8]. HbE heterozygotes are usually asymptomatic with normal red cell indices; however, its combination with α- and β-thalassemia results in a wide variety of clinical manifestations [7]. Prothrombotic hemostatic abnormalities with an increased risk of thromboembolic events are recognized complications of these hemoglobinopathies [6].
There have been reports of thromboembolic events in individuals with HbE thalassemias. Wong et al reported two cases of cerebral ischemia secondary to extracranial occlusion of the carotid arteries in patients with HbE β-thalassemia [9]. Both occurred in patients less than 35 years. Another study reported recurrent thromboembolic events in a HbE β-thalassemia patient with increased levels of platelet factor 4 following splenectomy [10]. Other reports of thromboembolic events in individuals with thalassemia have emerged [11, 12]. The mechanism for the prothrombotic state in HbE thalassemia includes chronic platelet activation, elevated serum levels of endothelial adhesion proteins, increased activated monocyte- and granulocyte-mediated endothelial injury, low levels of proteins C and S, and increased cohesiveness of abnormal red blood cells [6].
Besides hemoglobinopathies, surgery, acute-intraabdominal inflammatory disorders, and malignancies increase the risk of MVT [3]. Surgery and acute intraabdominal inflammatory disorders such as inflammatory bowel disease (IBD), acute pancreatitis, and acute appendicitis create an imbalance between thrombogenesis and fibrinolysis by promoting the expression of tissue factor and prothrombotic cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 while reducing fibrinolysis via increased activity of plasminogen activator inhibitor-1 and downregulation of tissue plasminogen activator expression [13].
Malignancies are associated with a five- to seven-fold increase in the risk of venous thrombosis and are the leading identifiable cause of MVT [14, 15]. Malignancy-associated thrombosis is multifactorial, with various tumor-specific mechanisms such as direct compression of mesenteric vessels, expression of procoagulant factors by tumor cells, and intravasation of tumor cells into mesenteric vessels [15]. The emergence of the JAK2 V617F assay, a marker of myeloproliferative neoplasms and its associated risk of thrombosis, highlights some of the new developments in elucidating the cause of thrombosis in individuals in which no risk factor can be found [16]. Other factors implicated in MVT include prothrombin 20210A mutation, methylenetetrahydrofolate reductase TT677 genotype, antithrombin III, protein C and S deficiencies, hyperhomocysteinemia, and factor V Leiden mutation [5]. These risk factors are only found in about 50% of cases of MVT with the remaining occurring in individuals with no obvious risk factors and are therefore described as primary or idiopathic MVT [3]. Idiopathic MVT is a diagnosis of exclusion requiring an extensive workup to eliminate potential risk factors for thrombosis. Our index case was extensively evaluated for these risk factors, but none was found.
MVT may be an asymptomatic incidental finding on abdominal imaging or could present with acute abdominal pain [2]. Symptomatic MVT progresses through mesenteric circulation hypertension, bowel wall edema, bowel ischemia, peritonitis, and septic shock with variable clinical manifestations at different stages of the disease. The initial symptoms are usually non-specific often leading to delay in establishing a diagnosis [17]. Abdominal pain is the most common presentation, described as out of proportion with physical examination findings [1]. This is useful in establishing a diagnosis, especially in individuals with pain arising from underlying intraabdominal inflammatory conditions. Other abdominal symptoms include nausea, vomiting, and gastrointestinal bleeding [3]. Fever, diffuse abdominal pain, rigidity, and guarding when present are suggestive of peritonitis complicating bowel ischemia which could progress to septic shock. When present, peritonitis and septic shock are poor prognostic factors in MVT [18].
Establishing a diagnosis for MVT involves the use of data from clinical, laboratory, and imaging findings. The presence of risk factors for venous thrombosis in a patient with abdominal pain that is out of proportion with physical examination findings should raise a suspicion of MVT [19]. Laboratory tests are usually of little benefit in establishing a diagnosis; however, they play an important role in identifying the underlying predisposing factors for MVT [3]. Findings such as leukocytosis and lactic acidosis when present are suggestive of bowel ischemia, although they do not correlate with disease severity [14]. Plain abdominal radiographs are often not helpful in uncomplicated MVT, although the presence of distended bowels, edematous bowel wall, and pneumatosis intestinalis are suggestive of bowel infarction, a life-threatening complication of MVT [15]. When present, free peritoneal gas is indicative of bowel perforation. Contrast-enhanced abdominal CT is the imaging modality of choice in the diagnosis of MVT. CT findings include filling defect(s) in the SMV and/or portal vein as well as vascular congestion [15]. The presence of mesenteric and portal vein gas is suggestive of bowel wall infarction [20]. Bowel wall changes found on CT include wall thickening, dilated fluid-filled bowel loops, and pneumatosis intestinalis suggestive of transmural infarct [21]. Increased hydrostatic pressure in the mesenteric circulation could result in ascites which is detectable on CT imaging. Doppler ultrasound can be used as a screening tool in the diagnosis of MVT; however, the yield can be affected by operator proficiency, intraabdominal gases, and body habitus [22]. Angiographic evaluation is highly sensitive in the diagnosis of MVT. However, it is invasive and is therefore not a first-line imaging modality in the diagnosis of MVT [1]. It is useful when CT imaging is inconclusive in an individual with a high pretest probability for MVT and in those who require endovascular therapy [23].
Once an imaging diagnosis of MVT is established, an attempt should be made to identify the predisposing factor for venous thrombosis. The patient should be evaluated for occult malignancies, and additional laboratory tests for JAK2 V617F mutation, prothrombin 20210A mutation, methylenetetrahydrofolate reductase TT677 genotype, antithrombin III, protein C and S deficiencies, hyperhomocysteinemia, and factor V Leiden mutation should be performed as indicated [3].
The immediate goal of medical intervention is to reestablish flow in the mesenteric venous system and prevent disease progression. Systemic anticoagulation with unfractionated or low molecular weight heparin should be initiated immediately after a diagnosis of MVT is established [5]. Studies have shown that prompt commencement of therapeutic anticoagulation results in high rates of recanalization [24, 25]. In one study, recanalization was achieved in 25 out of 27 patients with MVT or portal vein thrombosis on systemic anticoagulation [25]. Another study reported a recanalization rate of 73% in MVT and 39% when the portal vein is involved [24]. In that study, the presence of ascites was associated with an increased risk of failure of recanalization despite early anticoagulation. Individuals with thrombosis triggered by identifiable intraabdominal inflammatory conditions should be anticoagulated for 3 - 6 months to prevent a recurrence, while lifelong anticoagulation is recommended for those with non-modifiable hypercoagulable states and idiopathic MVT [3]. Additional supportive care in the management of acute MVT includes bowel rest, antibiotics, and serial evaluation with CT to monitor disease progression or resolution.
Invasive interventions such as surgery and interventional radiology-guided thrombolysis/thrombectomy are generally reserved for individuals with persistent symptoms or disease progression despite anticoagulation. Intravascular thrombolytic therapy or thrombectomy has been successfully used in the treatment of individuals who have worsening symptoms despite anticoagulation and are not ideal candidates for surgery [26]. However, there is an increased risk of bleeding from these procedures [26, 27]. In patients who can tolerate surgery, the presence of persistent symptoms with features of bowel infarction, peritonitis, or gut perforation despite anticoagulation is an indication for surgical intervention [3]. Resection of non-viable segments and end-to-end anastomosis of viable segments is standard surgical practice. Efforts should be made intraoperatively to determine gut viability to reduce the length of resected bowel and the risk of short bowel syndrome [28]. Both intraoperative and postoperative heparin therapies are important for positive outcomes [29]. The mortality rate following surgical intervention for MVT is about 20-50%, with the treatment outcome depending on the underlying cause of thrombosis, the extent of bowel infarction and resection as well as recurrence of thrombosis [3, 30].
Conclusion
Acute MVT can occur in individuals with HbE thalassemia in the absence of other known risk factors. The clinical findings are non-specific, therefore, a high index of suspicion is required for diagnosis. Contrast-enhanced abdominal CT confirms the diagnosis and medical management with systemic anticoagulation is the initial treatment of choice, while surgery and other invasive interventions are reserved for persistent symptoms or progressive disease with bowel infarction. The treatment outcome depends on the underlying risk factor for venous thrombosis, presence, and extent of bowel infarction as well as disease recurrence.
Learning points
Diagnosis of MVT requires a high index of suspicion and should be considered as a differential diagnosis in any individual presenting with acute abdomen.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient for publication of this case report and the accompanying images
Author Contributions
JA, AO, and MS conceived and designed the study. AO and MS collected and interpreted all relevant clinical and laboratory data. JA, AO, SO, MS and RS prepared the manuscript. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418.
doi pubmed - Sulger E, Dhaliwal HS, Goyal A, et al. Mesenteric Venous Thrombosis. [Updated Jul 22, 2021]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459184/.
- Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263.
doi pubmed - Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ, 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585-593.
doi pubmed - Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62-69.
doi pubmed - Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99(1):36-43.
doi pubmed - Fucharoen S, Weatherall DJ. The hemoglobin E thalassemias. Cold Spring Harb Perspect Med. 2012;2(8):a011734.
doi pubmed - Vichinsky E. Hemoglobin e syndromes. Hematology Am Soc Hematol Educ Program. 2007:79-83.
doi pubmed - Wong V, Yu YL, Liang RH, Tso WK, Li AM, Chan TK. Cerebral thrombosis in beta-thalassemia/hemoglobin E disease. Stroke. 1990;21(5):812-816.
doi pubmed - van Teunenbroek A, Wijburg FA, ten Cate JW, van den Berg W, Weening RS. Thromboembolic complications in an asplenic HbE-beta-thalassaemia patient. Neth J Med. 1989;35(3-4):123-127.
- Michaeli J, Mittelman M, Grisaru D, Rachmilewitz EA. Thromboembolic complications in beta thalassemia major. Acta Haematol. 1992;87(1-2):71-74.
doi pubmed - Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G, Mannucci AP. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111(2):467-473.
doi pubmed - Lentz SR. Thrombosis in the setting of obesity or inflammatory bowel disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):180-187.
doi pubmed - Singal AK, Kamath PS, Tefferi A. Mesenteric venous thrombosis. Mayo Clin Proc. 2013;88(3):285-294.
doi pubmed - Rha SE, Ha HK, Lee SH, Kim JH, Kim JK, Kim JH, Kim PN, et al. CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics. 2000;20(1):29-42.
doi pubmed - Vannucchi AM. JAK2 mutation and thrombosis in the myeloproliferative neoplasms. Curr Hematol Malig Rep. 2010;5(1):22-28.
doi pubmed - Yoon SH, Lee MJ, Jung SY, Ho IG, Kim MK. Mesenteric venous thrombosis as a complication of appendicitis in an adolescent: A case report and literature review. Medicine (Baltimore). 2019;98(48):e18002.
doi pubmed - Boley SJ, Kaleya RN, Brandt LJ. Mesenteric venous thrombosis. Surg Clin North Am. 1992;72(1):183-201.
doi - Choudhary AM, Grayer D, Nelson A, Roberts I. Mesenteric venous thrombosis: a diagnosis not to be missed! J Clin Gastroenterol. 2000;31(2):179-182.
doi pubmed - Abboud B, El Hachem J, Yazbeck T, Doumit C. Hepatic portal venous gas: physiopathology, etiology, prognosis and treatment. World J Gastroenterol. 2009;15(29):3585-3590.
doi pubmed - Bartnicke BJ, Balfe DM. CT appearance of intestinal ischemia and intramural hemorrhage. Radiol Clin North Am. 1994;32(5):845-860.
- Reginelli A, Genovese E, Cappabianca S, Iacobellis F, Berritto D, Fonio P, Coppolino F, et al. Intestinal Ischemia: US-CT findings correlations. Crit Ultrasound J. 2013;5(Suppl 1):S7.
doi pubmed - Grisham A, Lohr J, Guenther JM, Engel AM. Deciphering mesenteric venous thrombosis: imaging and treatment. Vasc Endovascular Surg. 2005;39(6):473-479.
doi pubmed - Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, Heller J, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51(1):210-218.
doi pubmed - Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000;32(3):466-470.
doi pubmed - Hollingshead M, Burke CT, Mauro MA, Weeks SM, Dixon RG, Jaques PF. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16(5):651-661.
doi pubmed - Smalberg JH, Spaander MV, Jie KS, Pattynama PM, van Buuren HR, van den Berg B, Janssen HL, et al. Risks and benefits of transcatheter thrombolytic therapy in patients with splanchnic venous thrombosis. Thromb Haemost. 2008;100(6):1084-1088.
doi pubmed - Rhee RY, Gloviczki P, Mendonca CT, Petterson TM, Serry RD, Sarr MG, Johnson CM, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
doi - Marshad M, Maresch M, Al Abbasi T. Intraoperative catheter directed thrombolytic therapy for the treatment of superior mesenteric and portal Vein thrombosis. Int J Surg Case Rep. 2018;53:242-245.
doi pubmed - Divino CM, Park IS, Angel LP, Ellozy S, Spiegel R, Kim U. A retrospective study of diagnosis and management of mesenteric vein thrombosis. Am J Surg. 2001;181(1):20-23.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


