| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 6, June 2022, pages 274-280
Carfilzomib-Induced Thrombotic Microangiopathy: Focus on Pathogenesis
Odianosen Eigbire-Molena, Daniela Hermelina, Douglas Blackallb, c
aDepartment of Pathology, Saint Louis University School of Medicine, Saint Louis, MO 63104, USA
bDepartment of Pathology and Laboratory Services, Providence Health and Services, Portland, OR 97213, USA
cCorresponding Author: Douglas Blackall, Department of Pathology and Laboratory Services, Providence Health and Services, Portland, OR 97213, USA
Manuscript submitted March 18, 2022, accepted May 26, 2022, published online June 11, 2022
Short title: Thrombotic Microangiopathy With Carfilzomib
doi: https://doi.org/10.14740/jmc3932
| Abstract | ▴Top |
Drug-induced thrombotic microangiopathies present in similar fashion but have varied pathogenic mechanisms. Carfilzomib is an irreversible proteasome inhibitor. Since its initial approval as a single agent for the treatment of relapsed or refractory multiple myeloma in 2012, there have been increasing reports of carfilzomib-induced thrombotic microangiopathy. However, the mechanism of this disease process is not fully understood. Without treatment, there is a high likelihood of end-organ damage, especially in the kidneys, and death. In recent reports, the lifesaving role of eculizumab, a terminal complement inhibitor, in managing and further preventing end-stage renal disease has been described. In this article, we present a case of carfilzomib-induced thrombotic microangiopathy in a patient with multiple myeloma and discuss the pathogenesis of thrombotic microangiopathy in this setting.
Keywords: Carfilzomib; Thrombotic microangiopathy; Therapeutic plasma exchange; Eculizumab; Atypical hemolytic uremia syndrome
| Introduction | ▴Top |
Thrombotic microangiopathy (TMA) is a term used to describe a heterogenous group of disorders with the common theme of microangiopathic hemolytic anemia, non-immune thrombocytopenia, and end-organ damage. Drug-induced TMA is a rare cause of this disease process. The diagnosis requires ruling out other causes of TMA such as Shiga toxin-producing Escherichia coli-associated hemolytic uremic syndrome (STEC-HUS), atypical hemolytic uremic syndrome (aHUS), underlying conditions, and thrombotic thrombocytopenic purpura (TTP) with ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) activity > 10% [1].
STEC-HUS and TTP are often considered primary causes of TMA, while aHUS and TMA associated with an underlying disease can be considered secondary causes. In this classification scheme, the term aHUS is reserved for TMA due to uncontrolled activation of the alternative complement pathway [2] and is usually associated with a genetic defect in complement regulatory proteins [3, 4]. Pathogenic variants of complement regulatory genes for complement factor H (CFH), complement factor I (CFI), membrane cofactor protein (MCP), and C3 have been described in aHUS [2-6]. Other secondary causes of TMA can result from a variety of conditions such as infection, cancer, hematopoietic stem cell transplantation, pregnancy, and medication exposure. However, it has been suggested that complement dysregulation may play a critical role in the acute stages of many secondary TMAs, not solely in aHUS [6].
Carfilzomib is a second-generation proteasome inhibitor approved by the US Food and Drug Administration (FDA) in 2012 as a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy [7]. Since that time, increasing reports of TMA secondary to carfilzomib have been published [8-27]. The most important step in the management of carfilzomib-induced TMA is stopping the drug. More recently, therapeutic success has been reported with the terminal complement inhibitor, eculizumab [8-14]. Additionally, plasmapheresis can be used as a temporizing measure until TTP is ruled out or when eculizumab is not available.
We present a case of TMA secondary to carfilzomib, describe the pathogenesis of TMA in this setting, and discuss possible links between the mechanism of proteasome inhibitors and complement dysregulation.
| Case Report | ▴Top |
Investigations
A 62-year-old African American woman with a long-standing history of essential hypertension was diagnosed with IgA kappa multiple myeloma. The diagnosis was made after she presented with a history of left hip pain that did not improve with conservative management. After a computed tomography (CT) scan of the hip showed lytic lesions, a bone marrow biopsy confirmed the diagnosis. At that time, she had no evidence of kidney disease, and her blood pressure was controlled with amlodipine, losartan, and metoprolol succinate.
Therapy for multiple myeloma was initiated with carfilzomib, lenalidomide, and dexamethasone. The patient received two doses of each medication during the first cycle of this regimen. On day 6, she presented to a local hospital emergency department with dyspnea on exertion, tachycardia (102/min), and an elevated blood pressure (152/101 mm Hg). She was afebrile and all other vital signs were within normal limits. She was oriented to person, place, and time and had no evidence of bleeding or peripheral edema. She also did not endorse abdominal pain or any recent episodes of vomiting, nausea, or diarrhea, nor did she endorse exposure to sick contacts.
Diagnosis
A chest CT ruled out a pulmonary embolism. Of note, her admission laboratory tests demonstrated evidence of a probable microangiopathic hemolytic anemia with platelets of 2 × 103/µL, hemoglobin of 11.6 g/dL, haptoglobin of < 8 mg/dL, total bilirubin of 4.2 mg/dL, lactate dehydrogenase (LDH) of 1,757 U/L, and the presence of schistocytes on the peripheral blood smear. Furthermore, her urine was amber colored, and the urinalysis was positive for blood (3+) and protein (3+). Her creatinine was 1.3 mg/dL at that time. A direct antiglobulin test (DAT) was negative, and the fibrinogen was within normal limits. However, her D-dimer was elevated at 5.63 mg/L (Table 1).
 Click to view | Table 1. Laboratory Values on Presentation |
Treatment
At this point, carfilzomib-induced TMA was suspected, and therapy with this agent was discontinued. However, as TTP was also a consideration, ADAMTS13 activity was assessed. The patient was admitted to the hospital, and therapeutic plasma exchange (TPE) was initiated (1.0 plasma volume per day with frozen plasma replacement using the Spectra Optia apheresis instrument: Terumo BCT, Lakewood, CO). After a 3-day hospitalization, which included two TPE procedures, the patient experienced no improvement in either her baseline clinical symptoms or her laboratory values. Therefore, she was transferred to our institution for further management.
The patient received two additional TPE procedures with mild improvement in her hemolytic laboratory indices, but she experienced a sustained rise in her creatinine (4.6 mg/dL) and additional evidence of acute kidney injury (blood urea nitrogen (BUN) of 68 mg/dL). On day 5, ADAMTS13 activity resulted at 91% (≥ 67%). Complement levels were reduced: C3 of 83 mg/dL (82 - 193), C4 of 9 mg/dL (15 - 57), and total complement, CH50, of 14 U/mL (> 41). TPE was discontinued. With compelling evidence to support a diagnosis of aHUS, intravenous eculizumab was started on day 7 at a dose of 900 mg. The patient completed two cycles of eculizumab (weekly dosing) but did not receive additional therapy since her liver enzymes became elevated. However, her hemoglobin stabilized within 2 weeks. Of note, additional complement factor testing was within the normal reference range, including factor H of 283 µg/mL (160 - 412), factor B of 33 mg/dL (20 - 51), and factor I of 44.6 µg/mL (29.3 - 58.5). CD46 and factor H antibodies were not assessed. On hospital day 8, the patient’s creatinine plateaued. By day 14, she demonstrated marked improvement in laboratory markers of hemolysis and her clinical symptoms (Table 2, Figs. 1, 2). Throughout her admission, all infectious disease tests were negative.
 Click to view | Table 2. Hemolytic Indices During Hospital Admission |
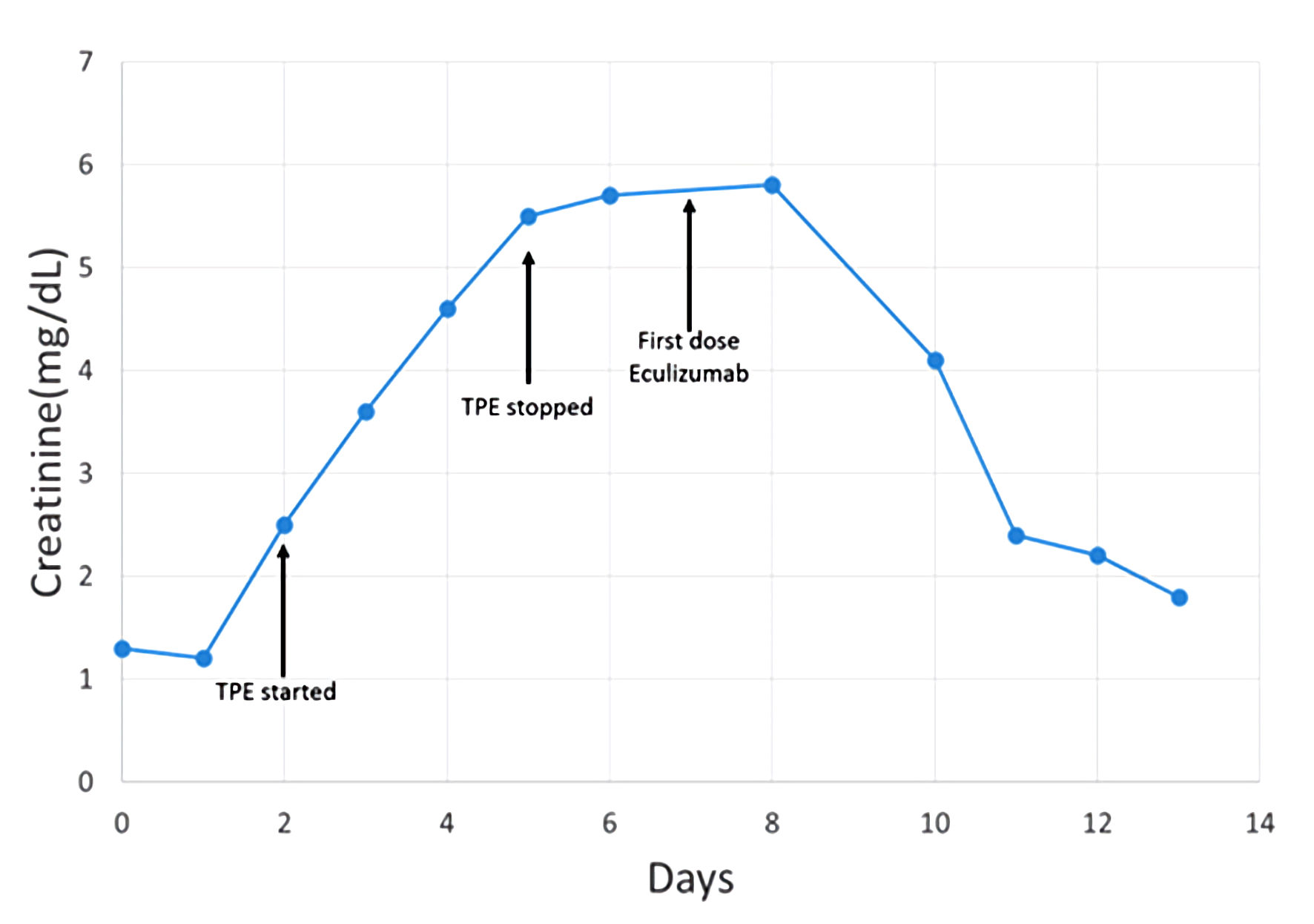 Click for large image | Figure 1. Patient timeline reflecting change in creatinine related to therapeutic interventions. TPE: therapeutic plasma exchange. |
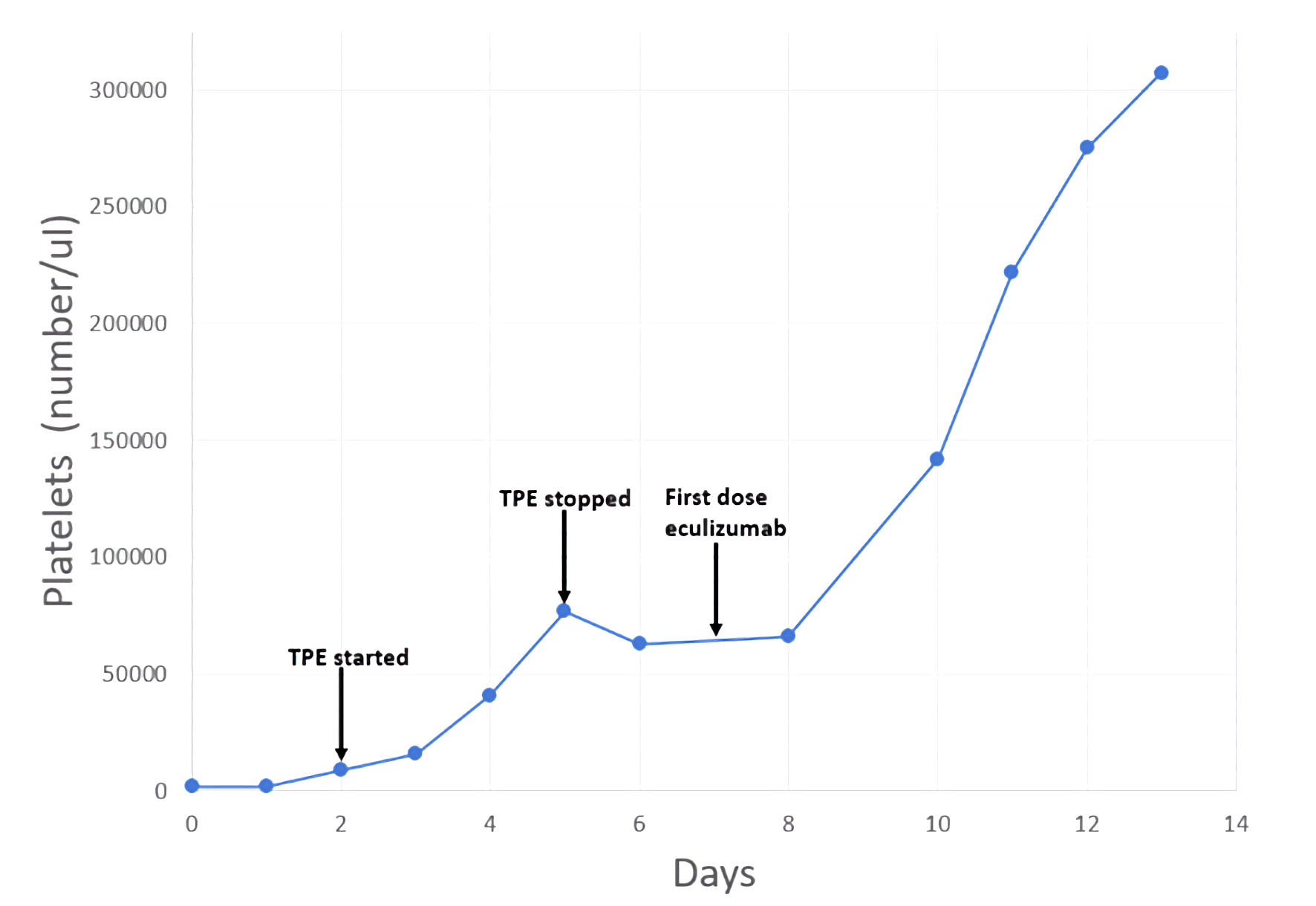 Click for large image | Figure 2. Patient timeline reflecting change in platelets related to therapeutic interventions. TPE: therapeutic plasma exchange. |
Follow-up and outcomes
After a 2-week hospitalization, the patient was discharged and was followed as an outpatient with the hematology/oncology clinic. After discharge, her hemolysis laboratory values and renal function continued to improve. Four months after this event, the patient underwent an autologous peripheral blood stem cell transplant and has been in recovery since that time.
| Discussion | ▴Top |
Carfilzomib-induced TMA is an uncommon but serious side effect in patients receiving carfilzomib for multiple myeloma. In a phase II study of single-agent carfilzomib in relapsed and refractory multiple myeloma, drug-associated TMA was not reported but drug-related anemia was reported in 46% of patients, thrombocytopenia was reported in 39% of patients, and acute kidney injury (AKI) was reported in 1.5% of patients [7]. A phase III study comparing carfilzomib and dexamethasone versus bortezomib and dexamethasone for relapsed multiple myeloma patients, the ENDEAVOR trial, reported TMA in two out of 463 patients [28]. The CARDAMON study, a phase II trial of carfilzomib, cyclophosphamide, and dexamethasone in untreated myeloma patients, reported TMA in eight out of 281 patients. ADAMTS13 activity was checked in seven of these eight patients, and all had normal activity [19]. Lastly, Fotiou et al reported TMA in six out of 114 patients (5%) with relapsed and refractory myeloma multiple myeloma who received carfilzomib. Of these six patients, ADAMTS13 activity was evaluated in two patients and was normal [17]. Therefore, carfilzomib-induced TMA must be on the differential diagnosis in a patient with multiple myeloma receiving carfilzomib who presents with anemia, thrombocytopenia, and AKI. In addition to the complete blood count (CBC) and comprehensive metabolic panel (CMP), a peripheral blood smear should be ordered to evaluate for schistocytes which would indicate active microangiopathy. Other hemolysis laboratory tests like haptoglobin and LDH should also be ordered. Finally, urinalysis should be performed to check for proteinuria.
Recognition of TMA is critical since stopping carfilzomib is the first step in management and can prevent further kidney damage. However, this can be challenging because other causes of TMA must be ruled out. Rarely, patients with multiple myeloma can develop aHUS due to myeloma progression [29], which should also be taken into consideration. Several factors that support carfilzomib-induced aHUS in our patient include a history of carfilzomib treatment, eventual improvement with discontinuation of carfilzomib, lack of recurrence of TMA in the absence of carfilzomib, and ruling out other causes of TMA.
There is no consensus definition for TMA. Although several authors have proposed diagnostic criteria for TMA, the histologic findings of TMA on biopsy remains the gold standard [30]. The kidney is the most often involved organ. However, renal biopsy is often not feasible due to the increased risk of bleeding from thrombocytopenia [30]. In this report, our diagnosis of TMA was based on the laboratory findings of anemia, thrombocytopenia, schistocytes on the peripheral blood smear, elevated LDH, negative direct and indirect Coombs tests, hypertension, and proteinuria. These findings would satisfy most criteria for TMA, including those recently proposed by Jodele et al [31].
Carfilzomib-induced TMA has been reported as early as 24 h and as late as 24 months after therapy [19]. In this report, our patient presented on cycle 1, day 6. Carfilzomib was immediately stopped and supportive care with plasmapheresis was initiated while evaluation for the cause of the TMA was undertaken. ADAMTS13 activity > 10% ruled out TTP. There was a low clinical suspicion for primary HUS. Given the patient’s history, the diagnosis of carfilzomib-induced TMA was made. Complement level test was performed but can be difficult to interpret because complement levels can be normal in TMA [32]. Genetic test for pathogenic complement gene variants and test for terminal complement activation (elevated sC5b-9 complex) was not performed in this case.
Recently, eculizumab has been identified as a lifesaving therapy for carfilzomib-induced TMA, especially in cases that do not improve after carfilzomib withdrawal [11, 33]. Rassner et al reported a case of two patients with carfilzomib-induced TMA that only improved with eculizumab [13]. Likewise, Portuguese et al noted that in two patients the early initiation of eculizumab reduced the progression of carfilzomib-induced TMA [9]. Our patient’s TMA appeared to be refractory to carfilzomib withdrawal and multiple apheresis procedures. There was no improvement in creatinine though there was some increase in the platelet count. However, the patient responded completely to eculizumab. There was a marked improvement in her renal function and platelet count, and her overall clinical picture improved within 2 weeks of eculizumab administration. The success of eculizumab in treating carfilzomib-induced TMA suggests a relationship between the mechanism of proteosome inhibition and complement pathway dysregulation.
Proteasome inhibitors are drugs that target the ubiquitin-proteasome pathway, which results in a blockage of cell cycle progression and an antiproliferative activity against many tumor types [34]. The transcription factor NF-κB is a regulatory protein whose mode of activation is controlled by the ubiquitin-proteasome pathway. In the resting cell state, NF-κB is bound to unphosphorylated IκB, thereby blocking the nuclear translocation of NF-κB. When triggered by proinflammatory cytokines, inflammation, or stress, a signal cascade occurs which activates a multisubunit IκB kinase (IKK). Activated IKK phosphorylates IκB leading to its degradation through ubiquitination. This releases free NF-κB which can then translocate into the nucleus and stimulate the transcription of many proteins including vascular endothelial growth factor (VEGF) [34]. Proteasome inhibitors prevent the ubiquitination of IκB, which prevents free release and activation of NF-κB and impairs the transcription of VEGF. Thus, a downstream effect of carfilzomib is inhibition of the VEGF pathway.
VEGF is most known for its critical role in angiogenesis, but a link between VEGF and complement regulation has been described. Keir et al demonstrated that VEGF inhibition decreases local CFH and other complement regulators in the eye and kidney through reduced VEGFR2/PKC-α/CREB signaling [35]. In that study, human retinal epithelial cells and renal podocytes with pathogenic complement regulatory gene variants showed more complement deposits compared to controls. Additionally, the deposits were increased by VEGF antagonism. These results suggest that VEGF plays a role in protecting the renal microvasculature from complement damage.
Moscvin et al present additional evidence that alternative complement pathway mutations may be a risk factor for carfilzomib-induced TMA in multiple myeloma patients [36]. In their abstract, deletions of the CFHR3-CFHR5 region were present in seven out of 10 cases of carfilzomib-induced TMA. Two heterozygous and four homozygous complement pathway gene deletions were reported. Although the sample size is small, their results suggest that mutations in the alternative complement pathway increase the risk of TMA in multiple myeloma patients receiving carfilzomib. Similar findings were reported by Portuguese et al who found heterozygous CFHR3-CFHR1 deletions in two patients with carfilzomib-induced TMA [9].
Taken together, these data suggest a multi-factorial mechanism for the pathogenesis of carfilzomib-induced TMA. The first risk factor is the multiple myeloma diagnosis, as multiple myeloma is an independent risk factor for TMA [19]. Therefore, we must consider the underlying malignancy in these patients as a contributing factor. The second risk factor is the presence of mutations in the alternative complement pathway. Early studies suggest that alternative complement pathway gene mutations may play a role in carfilzomib-induced TMA [9, 36]. The final contributing factor is exposure to carfilzomib. When carfilzomib is given, there is reduced local VEGF production by renal epithelial cells. This results in reduced local complement regulatory proteins, which makes endothelial cells vulnerable to complement activation. Any combination of the above risk factors would effectively create a pro-TMA environment.
Conclusions
Carfilzomib-induced TMA is an important adverse effect to recognize in patients receiving carfilzomib for multiple myeloma. Withdrawal of carfilzomib and treatment with eculizumab have proven successful in some patients. The mechanism of this adverse effect may be related to the compound effects of multiple myeloma, the presence of mutations in the alternative complement pathway, and the inhibitory effect of carfilzomib on VEGF, which results in local overactivation of complement in the glomerular endothelium. Further investigation into the mechanism of carfilzomib-induced TMA is required to better understand this rare but significant adverse effect.
Learning points
Carfilzomib-induced TMA should be in the differential diagnosis of a patient with multiple myeloma who is receiving carfilzomib and presents with anemia, thrombocytopenia, and AKI. The success of eculizumab in treating carfilzomib-induced TMA suggests a pathogenesis involving the complement pathway. Risk factors for TMA in multiple myeloma patients receiving carfilzomib include the underlying multiple myeloma, mutations in the alternative complement pathway, and local complement dysregulation due to the mechanism of carfilzomib.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
A waived informed consent form was approved according to our institution’s medical research requirements.
Author Contributions
DB formulated the paper and reviewed the manuscript. OE and DH did the literature review and wrote the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Fremeaux-Bacchi V, Kavanagh D, Nester CM, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2017;91(3):539-551.
doi pubmed - Zhang K, Lu Y, Harley KT, Tran MH. Atypical hemolytic uremic syndrome: A Brief Review. Hematol Rep. 2017;9(2):7053.
doi pubmed - Avila Bernabeu AI, Cavero Escribano T, Cao Vilarino M. Atypical hemolytic uremic syndrome: new challenges in the complement blockage era. Nephron. 2020;144(11):537-549.
doi pubmed - Afshar-Kharghan V. Atypical hemolytic uremic syndrome. Hematology Am Soc Hematol Educ Program. 2016;2016(1):217-225.
doi pubmed - Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681-696.
doi - Fakhouri F, Fila M, Provot F, Delmas Y, Barbet C, Chatelet V, Rafat C, et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol. 2017;12(1):50-59.
doi pubmed - Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, Trudel S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817-2825.
doi pubmed - Freyer CW, Bange EM, Skuli S, Hsu M, Lin J, Cuker A, Cohen AD, et al. Carfilzomib-Induced Atypical Hemolytic Uremic Syndrome in a Patient With Heterozygous CFHR3/CFHR1 Deletion Treated With Eculizumab. Clin Lymphoma Myeloma Leuk. 2021;21(11):e845-e849.
doi pubmed - Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446.
doi pubmed - Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher. 2015;30(5):308-310.
doi pubmed - Moliz C, Gutierrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88.
doi - Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case Report: Carfilzomib-induced Thrombotic Microangiopathy With Complement Activation Treated Successfully With Eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157.
doi pubmed - Rassner M, Baur R, Wasch R, Schiffer M, Schneider J, Mackensen A, Engelhardt M. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with Eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32.
doi pubmed - Gosain R, Gill A, Fuqua J, Volz LH, Kessans Knable MR, Bycroft R, Seger S, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930.
doi pubmed - Jindal N, Jandial A, Jain A, Lad D, Prakash G, Khadwal A, Nada R, et al. Carfilzomib-induced thrombotic microangiopathy: A case based review. Hematol Oncol Stem Cell Ther. 2020.
doi pubmed - Blasco M, Martinez-Roca A, Rodriguez-Lobato LG, Garcia-Herrera A, Rosinol L, Castro P, Fernandez S, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187.
doi pubmed - Fotiou D, Roussou M, Gakiopoulou C, Psimenou E, Gavriatopoulou M, Migkou M, Kanellias N, et al. Carfilzomib-associated renal toxicity is common and unpredictable: a comprehensive analysis of 114 multiple myeloma patients. Blood Cancer J. 2020;10(11):109.
doi pubmed - Monteith BE, Venner CP, Reece DE, Kew AK, Lalancette M, Garland JS, Shepherd LE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e800.
doi pubmed - Camilleri M, Cuadrado M, Phillips E, Wilson W, Jenner R, Pang G, Kamora S, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760.
doi pubmed - Yui JC, Van Keer J, Weiss BM, Waxman AJ, Palmer MB, D'Agati VD, Kastritis E, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-352.
doi pubmed - Darwin A, Malpica L, Dhanoa J, Hashmi H. Carfilzomib-induced atypical haemolytic uraemic syndrome: a diagnostic challenge and therapeutic success. BMJ Case Rep. 2021;14(2):e239091.
doi pubmed - Atrash S, Joiner A, Barlogie B, Medlin S. Fatal thrombotic microangiopathy developing within 24 hours of carfilzomib in a patient with relapsed multiple myeloma (MM). Blood. 2012;120(21):5037.
doi - Chen Y, Ooi M, Lim SF, Lin A, Lee J, Nagarajan C, Phipps C, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450.
doi pubmed - Haddadin M, Al-Sadawi M, Madanat S, Tam E, Taiwo E, Luhrs C, McFarlane SI. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243.
doi pubmed - Hobeika L, Self SE, Velez JC. Renal thrombotic microangiopathy and podocytopathy associated with the use of carfilzomib in a patient with multiple myeloma. BMC Nephrol. 2014;15:156.
doi pubmed - Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636.
doi pubmed - Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: A cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404.
doi pubmed - Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R, Facon T, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38.
doi - Portuguese AJ, Gleber C, Passero FC, Jr., Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195.
doi pubmed - Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021;56(8):1805-1817.
doi pubmed - Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, Myers K, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645-653.
doi pubmed - Gavriilaki E, Anagnostopoulos A, Mastellos DC. Complement in thrombotic microangiopathies: unraveling Ariadne's thread into the labyrinth of complement therapeutics. Front Immunol. 2019;10:337.
doi pubmed - Cavero T, Rabasco C, Lopez A, Roman E, Avila A, Sevillano A, Huerta A, et al. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol Dial Transplant. 2017;32(3):466-474.
doi pubmed - Myung J, Kim KB, Crews CM. The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev. 2001;21(4):245-273.
doi pubmed - Keir LS, Firth R, Aponik L, Feitelberg D, Sakimoto S, Aguilar E, Welsh GI, et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J Clin Invest. 2017;127(1):199-214.
doi pubmed - Moscvin M, Liacos CI, Chen T, Theodorakakou F, Fotiou D, Regan E, Kastritis K, et al. Mutations in the alternative complement pathway in multiple myeloma patients with carfilzomib-induced thrombotic microangiopathy. Blood. 2021;138(Supplement 1):2708.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


