| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 8, August 2022, pages 369-373
A Rare Metastatic Primary Rectal Melanoma in a Geriatric Male
Onyinye Ugonaboa, b, Mujtaba Mohameda, Ebubechukwu Ezeha, Joseph Simmonsa, Jonathan Cudaa, Shima Ghavimia
aDepartment of Medicine, Marshall University Joan C. Edwards School of Medicine, Huntington, WV 25701, USA
bCorresponding Author: Onyinye Ugonabo, Internal Medicine Residency Program, Marshall University Joan C. Edwards School of Medicine, Huntington, WV 25701, USA
Manuscript submitted May 20, 2022, accepted July 14, 2022, published online August 19, 2022
Short title: Rare Metastatic PRM in Male
doi: https://doi.org/10.14740/jmc3929
| Abstract | ▴Top |
Primary rectal melanoma (PRM) is an uncommon malignancy whose etiology remains unknown. Most patients present with rectal bleeding. Distant metastasis is commonly seen in the lung and liver. The incidence rates for locoregional lymph node metastases on initial presentation are almost 60%. Histology and immunochemistry are useful and are the gold standard for diagnosis. The prognosis is very poor due to the late presentation of patients. Optimum surgical treatment remains controversial. Abdominoperineal resection was considered traditionally but over time, has been found to have no survival benefit. Current literature and studies, therefore, recommend wide local excision. The beneficial effects of chemotherapy versus radiotherapy use are still debatable. Herein, we discuss a case of a 72-year-old Caucasian male with rectal bleeding found to have metastasized PRM.
Keywords: Primary rectal melanoma; Abdominoperineal resection; Wide local excision
| Introduction | ▴Top |
Malignant melanoma is derived from the melanocytes and commonly involves the skin. Aside from the skin, malignant melanoma has been observed in the eyes, gastrointestinal tract, head, and genital regions. The rectal region is the most frequently affected part in gastrointestinal malignant melanoma. These mucosal tumors, unlike the skin, derive their origin from the melanocytes in the non-keratinized squamous epithelium. Malignant melanoma of the rectum is an extremely rare and very aggressive tumor [1]. It constitutes only 0.5-4% of all anorectal malignancies and less than 1% of all melanomas. Most patients present usually in the fifth or sixth decade of life [2, 3]. Some cases have reported an association between rectal melanoma and Caucasian women; however, due to inadequate population-based studies, this remains inconclusive. The common presenting symptoms are rectal bleeding, anal mass, and changes in bowel habits [4]. PRM is lethal with a median survival of 24 months and 5-year survival of 10% [5].
| Case Report | ▴Top |
Investigations
A 72-year-old Caucasian male widower, with a past medical history of an alcohol use disorder, presented to the emergency department with a chief complaint of bleeding per rectum, anal mass, and diarrhea for 11 months associated with anal pruritus and tenesmus. He denied any personal or family history of cancer. Significant findings on physical examination included a frail appearing elderly man with a distended abdomen, and a soft, tender, friable mass protruding from the anus. The skin was negative for any abnormal pigmentation or lesion. Significant laboratory findings included hemoglobin of 9.8 g/dL (reference range of 14 - 18 g/dL), platelet of 247 × 103/mm3 (150 - 440 × 103/mm3), mean corpuscular volume (MCV) of 75.7 fL (80 - 100 fL), albumin of 2.8 g/dL (3.1 - 4.5 g/dL), aspartate transaminase/alanine transaminase (AST/ALT) of 28/21 U/L (15 - 37/12 - 78 U/L), serum iron of 18 µg/dL (65 - 175 µg/dL), ferritin of 17.9 ng/mL (22 - 322 ng/mL), and iron saturation of 6.2% (20-55%).
Diagnosis
The patient had a contrast-enhanced computed tomography (CECT) of abdomen which showed numerous hepatic lesions, with the largest measuring 2.4 cm in diameter, a large soft tissue mass at the anorectal junction with bilateral inguinal lymphadenopathy. Colonoscopy revealed a mass at the rectum with friable mucosa, measuring about 3 cm (Fig. 1).
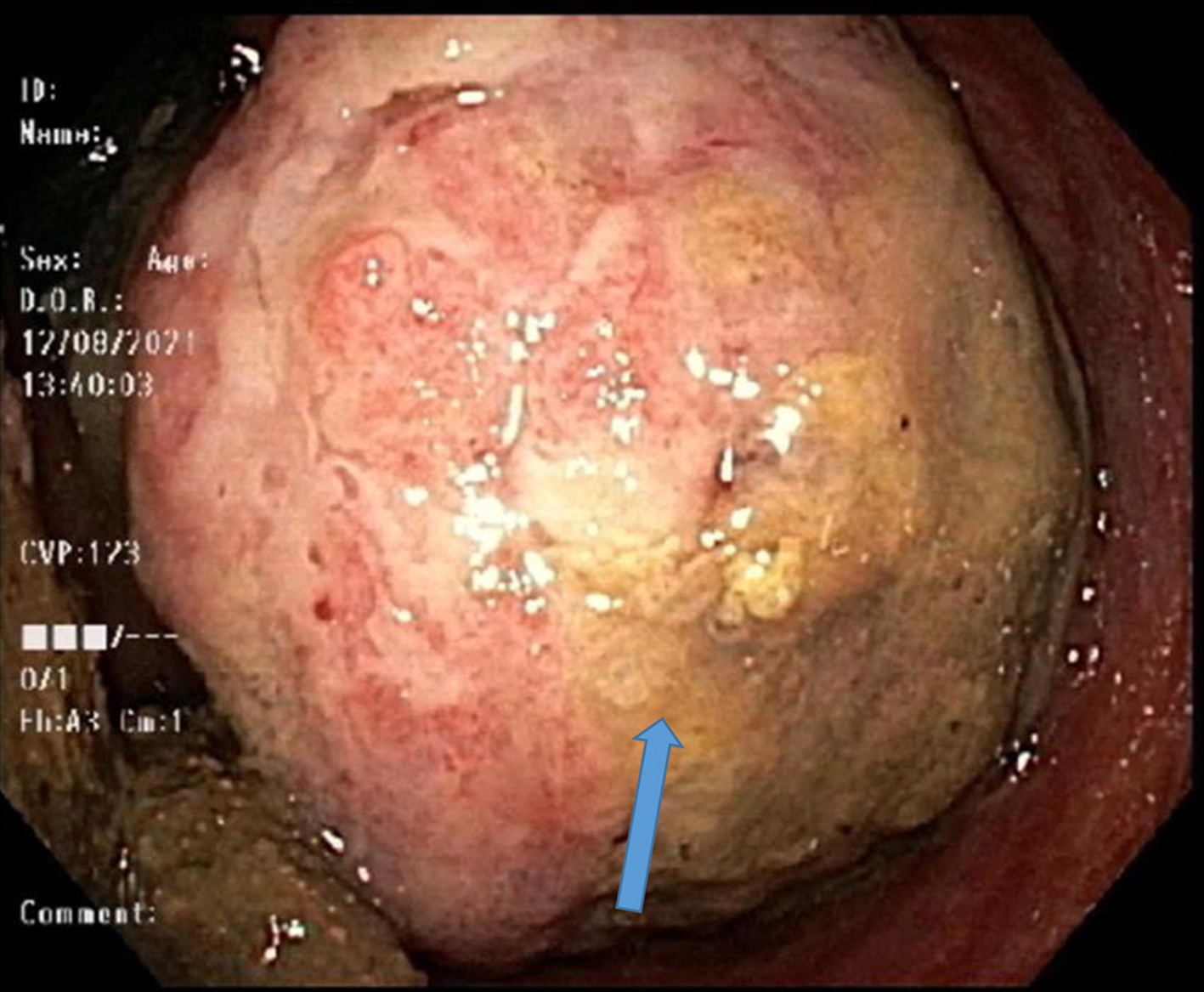 Click for large image | Figure 1. Colonoscope retroflection in rectum revealing rectal mass (blue arrow). |
The result of the biopsy showed a malignant proliferation of atypical pleomorphic cells with cherry-red macronuclei and amphophilic cytoplasm containing pigment (Fig. 2). The neoplastic cells were positive for Sox10 and S100, confirming the diagnosis of malignant melanoma.
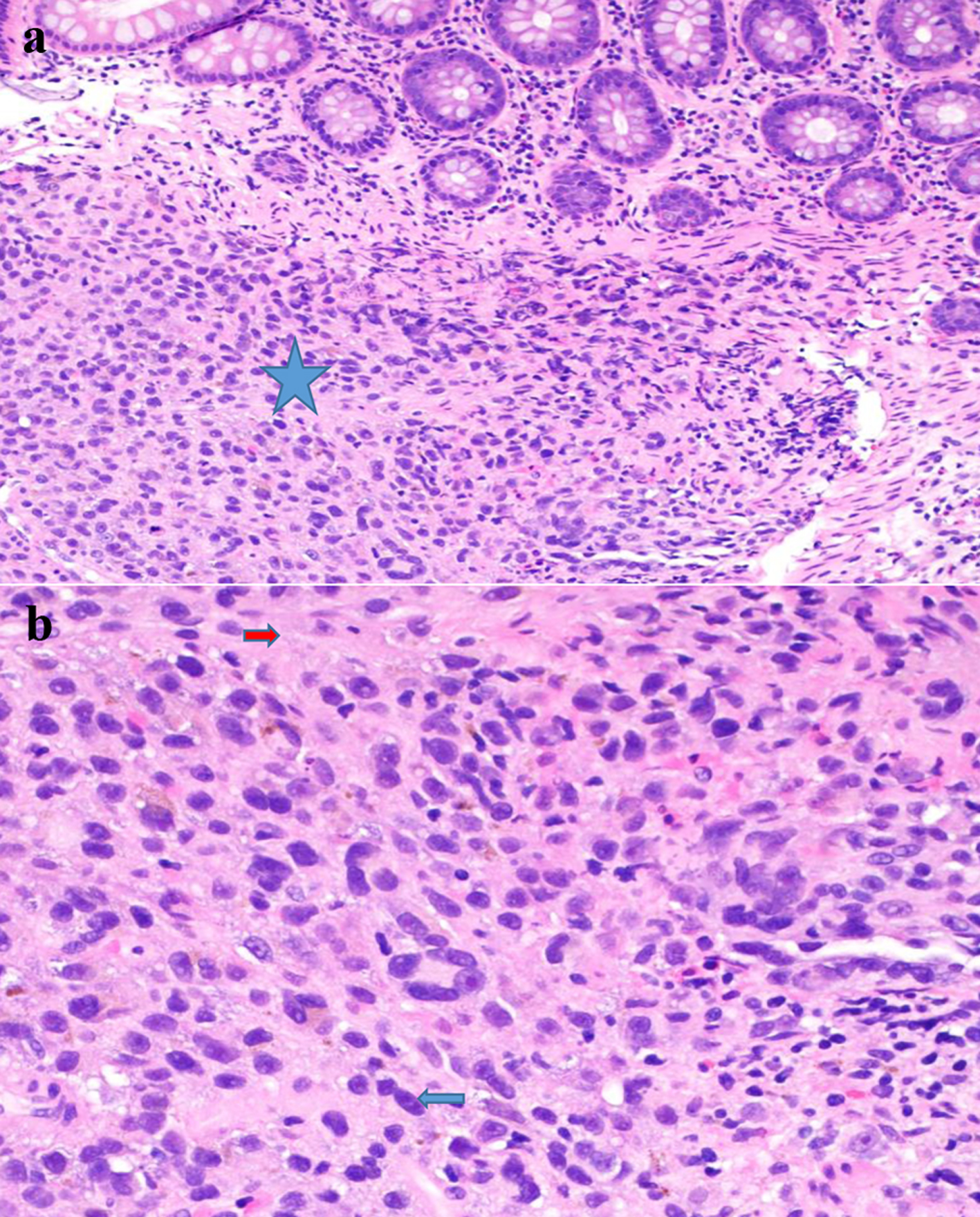 Click for large image | Figure 2. (a) Submucosal melanoma (blue star) (H&E stain, × 200). (b) The proliferation of markedly atypical melanocytes with prominent macronuclei (blue arrow) and cytoplasmic pigment (red arrow) (H&E stain, × 400). H&E: hematoxylin and eosin. |
Treatment
Genetic testing was not done as the patient declined it. Abdominoperineal resection (APR) surgery with adjuvant chemotherapy was discussed with the patient but he opted for palliative care with no intent for surgery or chemotherapy. The patient died 5 months after diagnosis.
| Discussion | ▴Top |
PRM is a type of melanoma arising from melanocytes in the rectal mucosa, more than 4 cm from the anal verge. This constitutes the primary difference from anorectal melanoma, which develops at or near the squamocolumnar junction [6]. PRM is a rare entity and not well documented in case reports and literature reviews. Most cases are misdiagnosed as hemorrhoids, adenocarcinoma, polyp, and rectal ulcer. Diagnosis is difficult for most clinicians due to its low incidence, non-specific symptoms, lack of melanin pigmentation, and histologic similarities with other cancers like lymphoma and sarcoma [7]. Most cases reported a higher incidence of anal melanoma compared to PRM. Goldman et al [8] reported 49 cases of primary anorectal melanoma among 24,323 patients with anorectal tumors in the total Swedish population between 1970 and 1984. Of these 49 tumors, 45 (91%) were located at or near the anorectal junction, three at the anal verge (6%), and only one in the rectum (3%) approximately 8 cm from the anal orifice [7]. Brady et al reported in another study [9] that summarized the largest series of PRM in the literature at Memorial Sloan Kettering Cancer Center from 1929 to 1993. This retrospective review reported 85 patients who were considered to have primary anorectal melanoma. Seven patients (8%) had tumors that were considered primary rectal malignant melanoma, while 78 patients (92%) had tumors that arose in the anal canal or the anal margin. In our case report, our patient had PRM arising 5 cm from the anus with no other cutaneous lesion detected at the time of diagnosis.
The cause of mucosal melanoma remains unidentified. However, some reports have found the Caucasian race to be a predisposing factor. The rate of mucosal melanoma remains twice as higher in Caucasians compared to African Americans [3]. Immunosuppression whether it is due to medications or infections such as human immunodeficiency virus (HIV) is associated with a high risk of melanoma. In a review of 619 patients who received cardiothoracic transplants, there was a 65-fold increased risk of cutaneous squamous cell carcinoma and a threefold increase in the risk of malignant melanoma [10]. Two susceptibility genes, CDKN2A and CDK4, have been identified to be associated with melanoma. In familial melanomas, a mutation in the CDKN2A gene, on the short arm of chromosome 9, increases the risk of melanoma. This is a tumor suppressor gene that encodes tumor suppressor proteins p16 and p19. Mutations in this gene have been found in 25% of familial melanomas [11, 12]. In addition, BRAF mutations are frequently reported in nevi and malignant melanomas. The frequency of BRAF mutation in mucosal melanomas is low compared to that of primary cutaneous melanoma [13, 14]. In our patient, there was no family history of melanoma. Genetic testing was not done as he declined it. PRM has a high tendency of spreading with the most common sites for metastases being inguinal lymph nodes, mesenteric lymph nodes, hypogastric lymph nodes, para-aortic lymph nodes, liver, lung, skin, and brain [1]. About 20% of recently diagnosed PRM patients are positive for lymph node disease in the inguinal region [15]. Distant metastases are identified in about 26-38% of patients [1, 2].
Biopsy through colonoscopy or proctoscopy is the gold standard to establish the diagnosis and staging of tumor extent. CECT and magnetic resonance imaging aid the characterization and extent of the tumor. A positron emission tomography scan can be used for lesions that are indeterminate on CECT. Histology and immunochemistry aid in the confirmation of diagnosis. Useful markers commonly used include S100 protein, Sox10, HMB 45, melan A, and microphthalmia transcription factor (MITF) [16, 17]. Our patient’s biopsy was positive for Sox10 and S100.
PRM is staged on a clinical basis, focusing on locoregional and distant metastasis. Stage I is a local disease involving two categories (stage IA with a depth of 0.75 mm, stage IB with a depth 0.75 - 1.5 mm), stage II has increased thickness with ulceration and has two substages (stage IIA with a depth of 1.5 - 4 mm, stage IIB > 4 mm), stage III involves regional lymph nodes, and stage IV shows distant metastatic disease [2].
There is no consensus at this moment on which surgical approach is preferred in the treatment of malignant melanoma of the rectum. Traditionally, APR was recommended due to its ability to control lymphatic spread predominantly to mesenteric nodes [18]. Most recent studies however suggest a less aggressive wide local excision (WLE). Combining radiation therapy with WLE has shown a decreased risk of local recurrence associated with this procedure. In a study carried out at the University of Texas MD Anderson Cancer Center, 54 patients who had WLE with radiotherapy between 1990 and 2008 had an excellent 5-year rate of local control with no significant improvement in survival rate [19]. APR is usually indicated for bulky tumors especially those with > 4 mm thickness, extensive tumors involving the anal sphincter that is not amenable to local excision [20]. Some studies have shown that APR is associated with increased mortality, especially in those with lymph node-positive disease. Nusrath et al [21] conducted a study on 30 patients with anorectal malignant melanoma, 15 had APR with a median survival of 13 months while five patients who had a WLE had a median survival of about 36 months. This was attributed to larger tumor size (3.5 cm) and positive nodal disease in the APR group compared to WLE. A retrospective study by Pessaux et al [18] showed no significant difference in survival between those that had APR versus WLE. This was attributed to the small sample size. WLE however was recommended for patients with easily achievable negative surgical margin and APR, for those with extensive disease involving the anal sphincter. In a meta-analysis study conducted by Smith et al [22], no oncological benefit was found between APR and WLE but recommended WLE with regular surveillance in most patients because of increased morbidity associated with APR. In some cases, surgical procedure is combined with adjuvant chemotherapy and radiotherapy in metastatic disease to achieve a good response. Chemotherapeutic agents used include cisplatin (CDDP), vinblastine (VB), dacarbazine (DTIC), interferon B (IFN), and interleukins (ILs)-2-8. According to a retrospective study by Kim et al [23], the majority of patients who received combination therapy with CDDP/DTIC/VB/IFN showed a major response with a median survival of 12.2 months, and only one with a complete response had survival duration of about 43 months. Our patient had a tumor size of 3 cm with distant metastasis to the liver, reflecting stage IV disease. The benefit of APR with chemotherapy was discussed with him but he declined further treatment and opted for palliative care.
Conclusion
The rarity of PRM and the limited number of patients who present early have prevented definitive trials examining the optimal treatment of curable rectal melanoma. Most patients however die irrespective of the therapy chosen due to the rapid tumor progression. Clinicians are therefore advised to carry out an appropriate investigation of any rectal mass encountered in practice.
Learning points
The takeaway point from this case is to make clinicians aware of primary rectal malignant melanoma and to consider it a differential diagnosis when evaluating patients with rectal bleeding with or without visible anal mass. PRM is an uncommon and aggressive disease that carries a poor prognosis. To enlighten readers about treatment modalities for PRM.
Acknowledgments
We would like to acknowledge our library clerk Mr. Frederick Price for supplying us with articles to complete this manuscript.
Financial Disclosure
This project was not supported by any grant or funding agency.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Informed Consent
The patient described in the case report had given informed consent for the case report to be published.
Author Contributions
Each author has individually been involved and participated in drafting the manuscript and revising it critically for important intellectual content and has given final approval of the version to be published. Each has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SG encouraged MM, OU to learn about PRM and its management. All authors discussed the medical literature. MM and OU presented the idea. OU wrote the manuscript with input from all authors.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- van Schaik PM, Ernst MF, Meijer HA, Bosscha K. Melanoma of the rectum: a rare entity. World J Gastroenterol. 2008;14(10):1633-1635.
doi pubmed - Singer M, Mutch MG. Anal melanoma. Clin Colon Rectal Surg. 2006;19(2):78-87.
doi pubmed - Row D, Weiser MR. Anorectal melanoma. Clin Colon Rectal Surg. 2009;22(2):120-126.
doi pubmed - Roviello F, Cioppa T, Marrelli D, Nastri G, De Stefano A, Hako L, Pinto E. [Primary ano-rectal melanoma: considerations on a clinical case and review of the literature]. Chir Ital. 2003;55(4):575-580.
- Maqbool A, Lintner R, Bokhari A, Habib T, Rahman I, Rao BK. Anorectal melanoma—3 case reports and a review of the literature. Cutis. 2004;73(6):409-413.
- Hazzan D, Reissmann P, Halak M, Resnick MB, Lotem M, Shiloni E. Primary rectal malignant melanoma: report of two cases. Tech Coloproctol. 2001;5(1):51-54.
doi pubmed - Falch C, Stojadinovic A, Hann-von-Weyhern C, Protic M, Nissan A, Faries MB, Daumer M, et al. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J Am Coll Surg. 2013;217(2):324-335.
doi pubmed - Goldman S, Glimelius B, Pahlman L. Anorectal malignant melanoma in Sweden. Report of 49 patients. Dis Colon Rectum. 1990;33(10):874-877.
doi pubmed - Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum. 1995;38(2):146-151.
doi pubmed - Wu E, Golitz LE. Primary noncutaneous melanoma. Clin Lab Med. 2000;20(4):731-744.
- Itin PH, Fistarol SK. [Genetic counseling and DNA testing in patients with increased risks for malignant melanoma]. Ther Umsch. 2003;60(8):469-472.
doi pubmed - Pho L, Grossman D, Leachman SA. Melanoma genetics: a review of genetic factors and clinical phenotypes in familial melanoma. Curr Opin Oncol. 2006;18(2):173-179.
doi pubmed - Shinozaki M, Fujimoto A, Morton DL, Hoon DS. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10(5):1753-1757.
doi pubmed - Edwards RH, Ward MR, Wu H, Medina CA, Brose MS, Volpe P, Nussen-Lee S, et al. Absence of BRAF mutations in UV-protected mucosal melanomas. J Med Genet. 2004;41(4):270-272.
doi pubmed - Droesch JT, Flum DR, Mann GN. Wide local excision or abdominoperineal resection as the initial treatment for anorectal melanoma? Am J Surg. 2005;189(4):446-449.
doi pubmed - Pham BV, Kang JH, Phan HH, Cho MS, Kim NK. Malignant melanoma of anorectum: two case reports. Ann Coloproctol. 2021;37(1):65-70.
doi pubmed - Prieto VG, Shea CR. Immunohistochemistry of melanocytic proliferations. Arch Pathol Lab Med. 2011;135(7):853-859.
doi pubmed - Pessaux P, Pocard M, Elias D, Duvillard P, Avril MF, Zimmerman P, Lasser P. Surgical management of primary anorectal melanoma. Br J Surg. 2004;91(9):1183-1187.
doi pubmed - Han J, Shi C, Dong X, Wang J, Wen H, Wang B, He Z. Laparoscopic abdomino-perineal resection for patients with anorectal malignant melanoma: a report of 4 cases. J Biomed Res. 2016;30(5):436-440.
- Reid A, Dettrick A, Oakenful C, Lambrianides A. Primary rectal melanoma. J Surg Case Rep. 2011;2011(11):2.
- Nusrath S, Thammineedi SR, Patnaik SC, Raju K, Pawar S, Goel V, Chavali RN, et al. Anorectal malignant melanoma-defining the optimal surgical treatment and prognostic factors. Indian J Surg Oncol. 2018;9(4):519-523.
doi pubmed - Smith HG, Glen J, Turnbull N, Peach H, Board R, Payne M, Gore M, et al. Less is more: A systematic review and meta-analysis of the outcomes of radical versus conservative primary resection in anorectal melanoma. Eur J Cancer. 2020;135:113-120.
doi pubmed - Kim KB, Sanguino AM, Hodges C, Papadopoulos NE, Eton O, Camacho LH, Broemeling LD, et al. Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer. 2004;100(7):1478-1483.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


