| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 5, May 2022, pages 229-234
A One-Year-Old Girl With Human Parvovirus B19 Infection and Hypocomplementemia Mimicking Incomplete Kawasaki Disease
Kazumi Ouraa, Shinichi Ishikawaa, Haruki Shiraishia, Yuji Maruoa, Norio Satoa, Takashi Suganumaa, Makoto Mikawaa, Tomonobu Satoa, b
aDepartment of Pediatrics, Kitami Red Cross Hospital, Kitami, Hokkaido 090-8666, Japan
bCorresponding Author: Tomonobu Sato, Department of Pediatrics, Kitami Red Cross Hospital, Kitami, Hokkaido 090-8666, Japan
Manuscript submitted February 21, 2022, accepted April 4, 2022, published online April 23, 2022
Short title: Parvovirus B19 Infection Mimicking KD
doi: https://doi.org/10.14740/jmc3917
| Abstract | ▴Top |
Human parvovirus B19 (B19) is a single-stranded DNA virus that targets erythroid progenitor cells in the bone marrow. B19 causes erythema infectiosum in children, transient aplastic anemia, pure red cell aplasia, hydrops fetalis, and contributes to other illnesses. An association between B19 infection and hypocomplementemia and rheumatoid arthritis has been reported, but the underlying mechanisms remain unclear. We report the case of a 1-year-old Japanese girl with persistent fever, skin rash, transient edema of the extremities, hypoalbuminemia, and hypocomplementemia associated with B19 infection. We considered Kawasaki disease (KD) and collagen diseases, particularly systemic lupus erythematosus, in our differential diagnosis. B19 infection might be associated with serological features that suggest systemic lupus erythematosus and may present with clinical symptoms seen in KD. Especially during erythema infectiosum epidemics, we must consider B19 infection in the differential diagnosis of KD patients who demonstrate atypical clinical symptoms and unexplained laboratory findings.
Keywords: Erythema infectiosum; Human parvovirus B19; Hypocomplementemia; Kawasaki disease; Systemic lupus erythematosus
| Introduction | ▴Top |
The human parvovirus B19 (B19) is a single-stranded DNA virus that targets erythroid progenitor cells that express CD36 antigen in the bone marrow [1]. B19 infection can either manifest with mild nonspecific symptoms or lead to acute disease that has a wide clinical spectrum, including erythema infectiosum (EI) in children, transient aplastic crisis in patients with congenital hemolytic disorders, and post infectious arthropathies similar to those of some collagen diseases, purpura, and nephropathies. In particular, it has been reported that B19 infection can cause fever, joint symptoms, and hypocomplementemia, as seen in systemic lupus erythematosus (SLE), with immune complex (IC) formation suspected to be involved in the underlying mechanism [2, 3].
Furthermore, some B19 infections present with symptoms that meet the diagnostic criteria for Kawasaki disease (KD), a vasculitis syndrome occurring in childhood [4, 5]. According to the American Heart Association guidelines outlined in 2004, incomplete KD is a term used in patients with less than four of the six diagnostic criteria for KD [6]. Early diagnosis and treatment are essential to prevent the sequelae of KD, but it often remains difficult to distinguish KD from the potential competing diagnoses when considering the differential diagnosis. As described above, patients with B19 infection might exhibit SLE-like symptoms or symptoms that meet the diagnostic criteria for KD, but it remains unclear whether B19 directly induces immunological mechanisms or other pathophysiological processes are involved. Therefore, clinicians have to distinguish these diseases based on patients’ symptoms and laboratory findings.
Herein, we report the case of a 1-year-old Japanese girl with persistent fever, skin rash, transient edema of the extremities, hypoalbuminemia, and hypocomplementemia to demonstrate the challenges in distinguishing B19 infection from KD and other collagen diseases, such as SLE.
| Case Report | ▴Top |
Investigations
A previously healthy 1-year-old Japanese girl developed fever and a skin rash 4 days prior to admission. Initially, the erythematous rash was found mainly on her limbs but then spread over her face and trunk. Her medical history was unremarkable, and she had no family history of collagen diseases. She was diagnosed with pharyngitis by her primary pediatrician and was treated with amoxicillin and a nonsteroidal anti-inflammatory drug. However, her symptoms did not resolve, and she was eventually referred to our department and admitted to our ward. At admission, 4 days after symptom onset, the child’s body temperature was 39.1 °C, and she had no significant arthralgia or hepatosplenomegaly. She demonstrated bilateral cervical lymphadenopathy, redness of the lips, and a punctate rash on the extremities, trunk, and face with a partial fusion tendency (Fig. 1a-c). She did not reveal conjunctival hyperemia of the eye, erythema of the palm, or redness at the site of her bacillus Calmette-Guerin vaccination. Her hematological tests were normal. The peripheral white blood cell count was 5.76 × 109/L, with 2.0% atypical lymphocytes (Table 1). No proteinuria or hematuria was observed. Blood biochemistry tests revealed increased levels of aspartate transaminase and lactate dehydrogenase and mild hyponatremia. In addition, increased levels of ferritin, soluble interleukin-2 receptor, and β2-microglobulin were found. Increased levels of D-dimer, indicating vasculitis, and N-terminal fragment of pro-B-type natriuretic peptide, a peptide secreted by cardiomyocytes during heart failure, were also observed. In contrast, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were not elevated. In the complement system, total hemolytic complement, C3, and C4 levels were low (Table 1). Anti-nuclear antibodies and anti-double-stranded DNA antibodies (anti-dsDNA Ab) were negative (Table 2). Total body enhanced computed tomography showed lymphadenopathy in the neck, axilla, and inguinal regions.
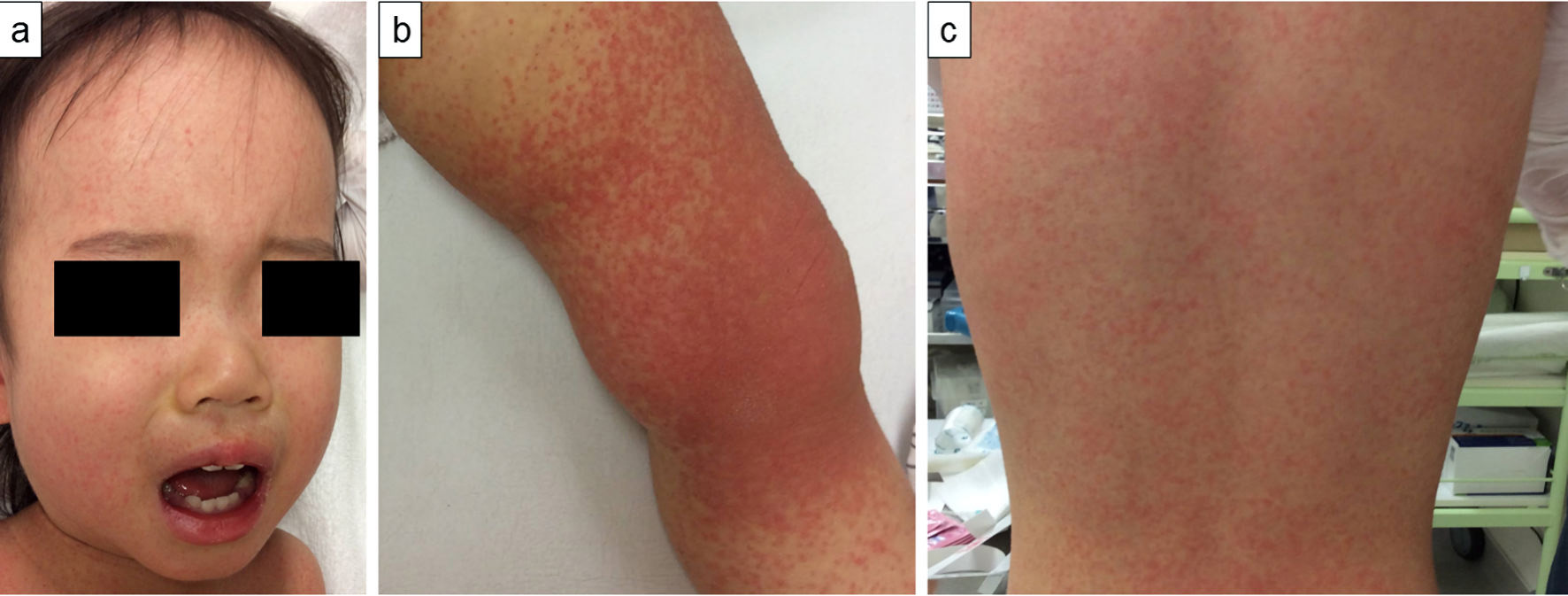 Click for large image | Figure 1. A 1-year-old Japanese girl with fever and skin rash. At admission, 4 days after symptom onset, photographs of patient’s skin demonstrated rash on her face (a), left knee (b), and back (c). A fine papular erythema is visible. |
 Click to view | Table 1. Laboratory Findings at Admission and 4 Days After Symptom Onset of a 1-Year-Old Japanese Girl With Fever and Skin Rash for 4 Days |
 Click to view | Table 2. Laboratory Findings at Admission of a 1-Year-Old Japanese Girl With Fever and Skin Rash for 4 Days |
Diagnosis
At admission, the patient’s clinical symptoms met four of the six diagnostic criteria for KD, although laboratory findings showed only mild elevation of acute phase reactants. Consequently, we assumed that her fever and skin rash resulted from a viral infection, and no intravenous immunoglobulin was administered. Based on her prolonged fever and the migrating skin rash with low CRP levels and hypocomplementemia, we also considered collagen disease, such as SLE. However, the blood tests, including the absolute lymphocyte count, ESR, and γ-globulin levels, were within normal limits, and anti-dsDNA Ab were negative. Therefore, the patient received fluid replacement therapy only and underwent repeated blood tests and ultrasonography to monitor the coronary arteries because we could not exclude incomplete KD. Subsequently, hypoproteinemia gradually progressed without proteinuria, and edema of the face and limbs occurred. The levels of pro-B-type natriuretic peptide as a disease marker of KD also increased. KD as a differential diagnosis was considered again as the cause of these abnormal laboratory findings. However, the patient’s blood tests, including leukocytes, thrombocytes, and CRP, were consistently within the normal range, and the D-dimer levels subsequently decreased to the normal range. Eight days after admission and 12 days after symptom onset, we received confirmation that anti-B19 immunoglobulin (Ig) M had been positive in the patient’s serum collected on admission.
Treatment
The patient received fluid replacement therapy only and underwent repeated blood tests and ultrasonography to monitor the coronary arteries because we could not exclude incomplete KD.
Follow-up and outcomes
Consecutively, the rash on her body gradually regressed and then changed to a lacy, reticulated appearance similar to that of EI (Fig. 2a, b). A blood test 11 days after symptom onset showed an increase in serum albumin and IgG, and complement levels returned to the normal range (Fig. 3). At this point, anti-B19 IgM and IgG were positive. The patient’s fever subsided on day 12 after symptom onset, and she was discharged on day 14. Neither desquamation of the fingers or toes nor a dilation of the coronary arteries was observed during the course of the disease.
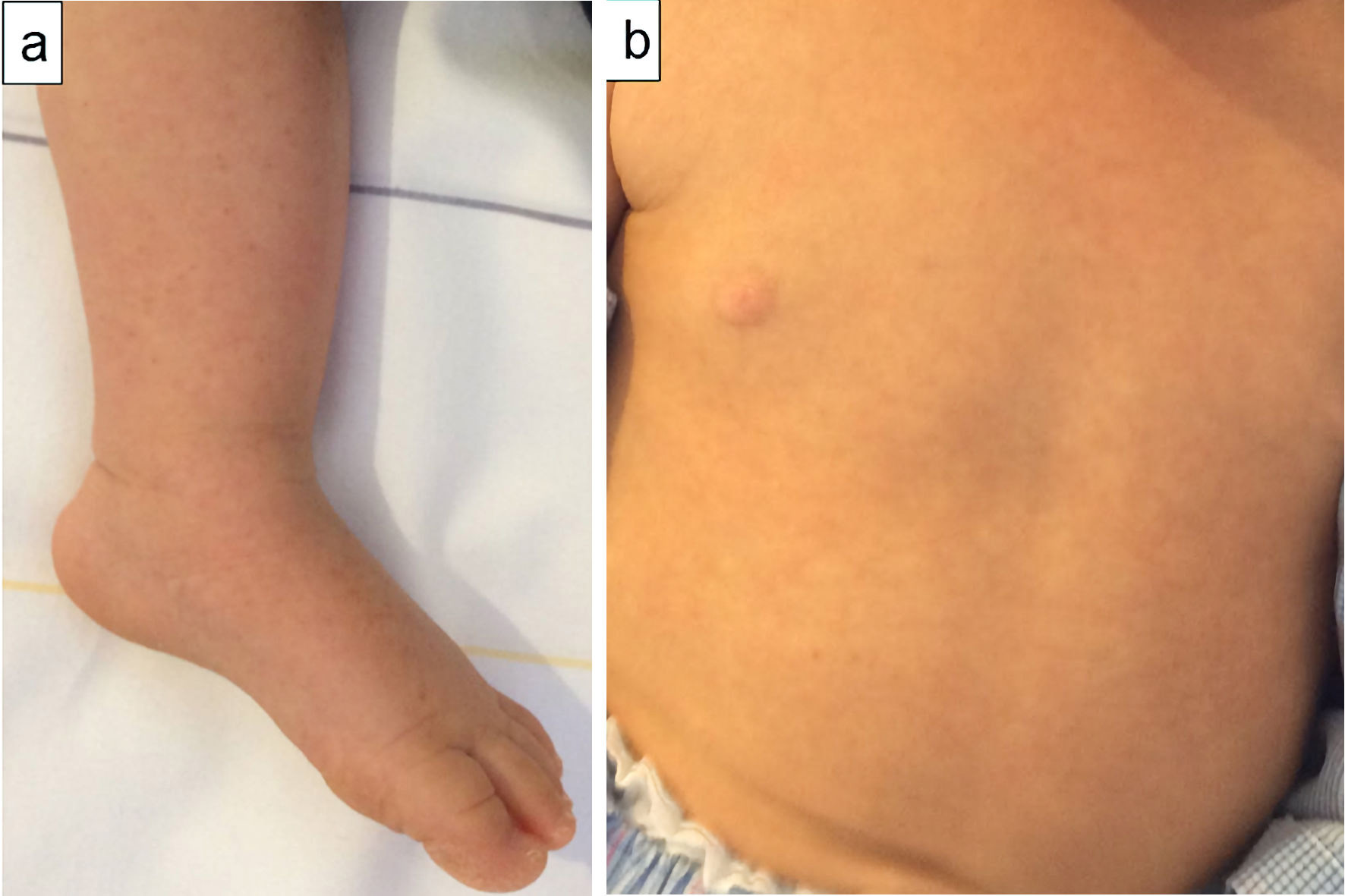 Click for large image | Figure 2. A 1-year-old Japanese girl with fever and skin rash. At discharge, 14 days after symptom onset, photographs of patient’s skin demonstrated rash on her foot (a) and trunk (b). The rash changed into a lacy, reticulated appearance similar to that of erythema infectiosum. |
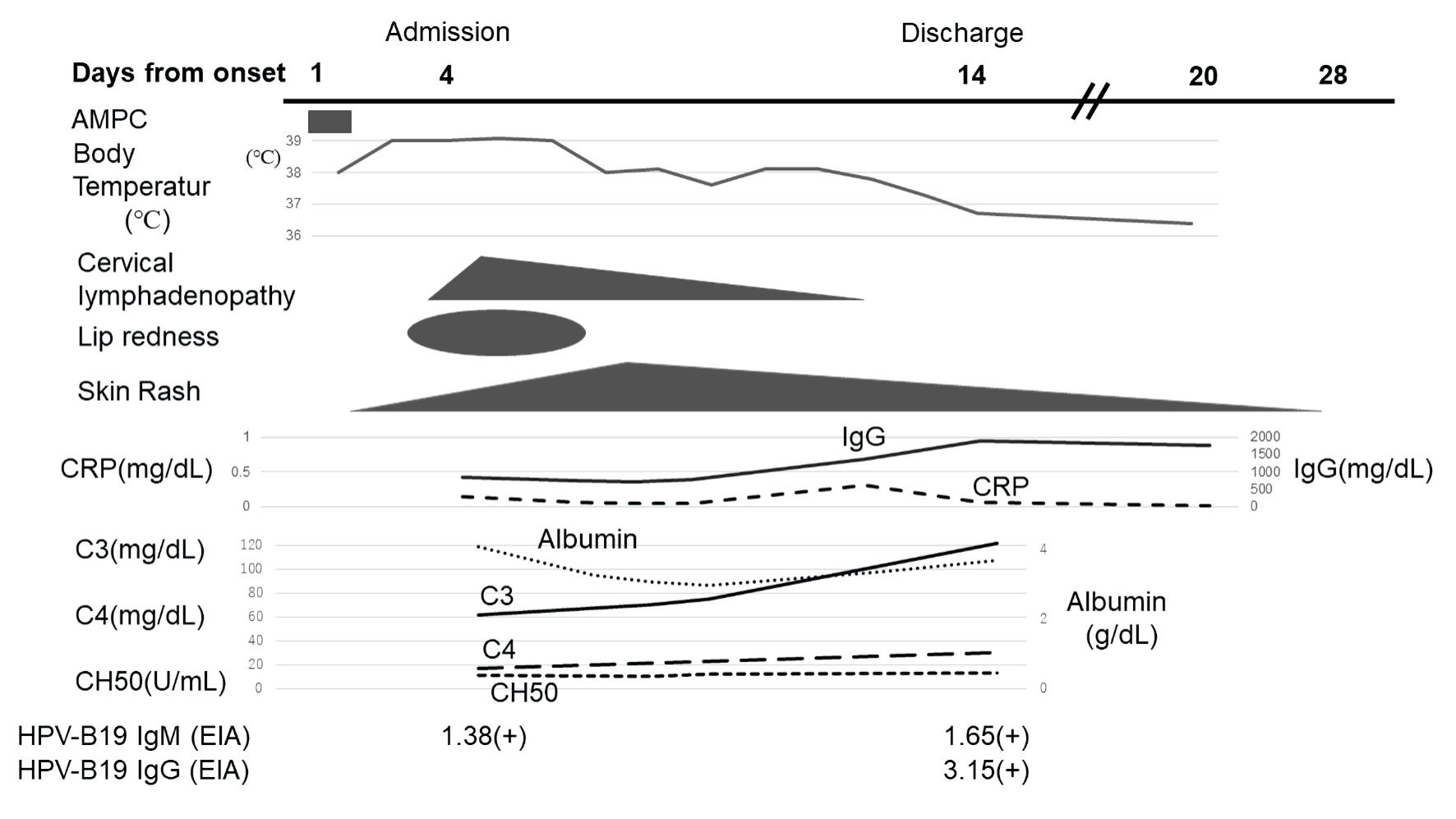 Click for large image | Figure 3. A 1-year-old Japanese girl with fever and skin rash for 4 days: clinical course in relation to symptoms and laboratory findings. The horizontal axis shows the course over time in days after the onset of the patient’s symptoms. AMPC: amoxicillin; CRP: C-reactive protein; CH50: total hemolytic complement; IgM: immunoglobulin M; IgG: immunoglobulin G; B19: human parvovirus B19; EIA: enzyme immunoassay. |
| Discussion | ▴Top |
In children, B19 infection is the well-known cause of EI. Its characteristics include fever, malaise, and initially a facial rash, which then spreads in the form of lace-like papules to the trunk and limbs. The rash usually disappears within a week, but it may recur for several months after exposure to sunlight or heat [7]. After the infection, B19 targets glycosphingolipid globosides, also known as blood group P antigens, to target erythroid progenitor cells in the bone marrow [8]. Although P antigens are abundantly expressed in erythroblasts, they are also expressed in mesoderm cells, such as vascular endothelial cells, synovial cells, and cardiomyocytes [8]. Hence, B19 infection causes various symptoms in organs throughout the body [9]. The pathophysiology of B19 infection is generally assigned to two components: direct tissue damage by B19 itself and immunological mechanisms, such as IC formation and serum complement system. Cases of B19 infection in which the complement system was activated by IC formation and resulted in hypocomplementemia have been reported [2-4]. Some authors stated that B19 infection with hypocomplementemia is associated with papular purpura, nephritis, and collagen disease-like symptoms seen in SLE [10, 11]. In particular, although the details of the underlying mechanism have not been unveiled, it has been reported that most patients with B19 infection presenting with SLE-like symptoms demonstrate hypocomplementemia [12]. Potential mechanisms, through which persistent B19 infection may cause hypocomplementemia and SLE-like symptoms, include abnormal activation of T cells due to upregulation of the major histocompatibility complex and costimulatory molecules on antigen-presenting cells [13, 14].
Our patient presented with fever, an atypical skin rash, cervical lymphadenopathy, and lip redness. She had hypocomplementemia suggestive of hypercytokinemia, for example, elevated levels of soluble interleukin-2 receptor, ferritin, and lactate dehydrogenase. In addition, hypoalbuminemia and hyponatremia, suggesting vascular hyperpermeability due to hypercytokinemia, were seen. A patient with B19 infection and hypocomplementemia who also demonstrated edema resulting from hypoalbuminemia has been reported previously [2]. In our case, although IC formation could not be demonstrated directly, low levels of both serum C3 and C4 suggested that classical pathways of the complement system might have been activated, potentially resulting in IC formation.
We needed to distinguish the B19 infection in our patient from collagen diseases, such as SLE. However, it is known that patients with SLE usually have positive anti-nuclear antibodies and autoimmune anti-dsDNA Ab, combined with high ESR but low CRP. Our patient had negative anti-nuclear antibodies and negative anti-dsDNA Ab and low ESR, and we consequently excluded SLE during the differential diagnosis.
Patients with KD often show elevated ESR and CRP levels and leukocytosis with neutrophilia, hyponatremia, hypoalbuminemia, and anemia [15]. In our case, fever, color changes in lip, skin rash, and cervical lymphadenopathy, but no conjunctival injection or changes in the hands and feet were observed. Furthermore, the patient had no elevated ESR or CRP, no leukocytosis, and did not develop dilation of the coronary arteries. Therefore, we also excluded KD. Finally, the positive anti-B19 IgM and IgG allowed the diagnosis of B19 infection.
Some patients with B19 infection may have symptoms matching the diagnostic criteria for KD [4, 6]. Fever and rash due to vasculitis may occur. Cervical lymphadenopathy, another diagnostic criterion for KD, also occurs in patients with B19 infection [16]. Of note, Nigro et al [4, 17] reported a case of KD with B19 infection and suggested that B19 infection might be the cause of KD. However, Yoto et al [18] and Chua et al [19] have subsequently refuted the idea that B19 infection may cause KD based on their virological examinations. On the other hand, it has been reported that some cases of B19 infection that met the diagnostic criteria for KD presented with vasculitis due to IC formation, and coronary arterial lesions were observed in a patient with KD and confirmed B19 infection [4]. Magro et al [20] and Takahashi et al [21] reported that the histology of patients with purpura due to B19 infection showed leukocytoclastic vasculitis, a finding in IC-induced angiopathy. Therefore, B19 infection might cause microangiopathy through IC formation. In the current case, a slight increase in D-dimer levels, but no dilation of the coronary arteries was observed. However, the complement levels suggested that microangiopathy caused by IC formation might have occurred.
B19 infection has been identified as a contributing factor in various diseases. Although B19 is unlikely to be the main cause of KD, it might play an auxiliary role in triggering or inducing inflammation in KD patients. Thus far, it is known that non-structural protein 1, produced by the B19 DNA, induces pro-inflammatory cytokine genes and the activation of inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, which are deeply involved in the pathophysiology of KD [22]. Since our patient presented with low CRP levels and no thrombocytosis, we did not administer high-dose gamma globulin and aspirin as the common therapy for KD patients but opted for careful follow-up. However, it remains unclear whether aggressive therapy is required in such cases.
Conclusions
In conclusion, clinicians need to be aware that B19 infection might demonstrate serological features suggesting SLE and present with clinical symptoms seen in KD. Especially during EI epidemics, we must consider B19 infection in the differential diagnosis of KD patients who demonstrate atypical clinical symptoms and laboratory findings. If any pathogen, especially B19, is detected in patients who meet the diagnostic criteria for KD and show severe inflammation or elevation of vasculitis markers, attention should be paid to the development of coronary artery lesions. No evidence exists on whether such cases require KD treatment. Regular follow-up, such as ultrasound cardiography, may be needed even if patients have low inflammatory markers. Therefore, more of these cases need to be reported.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that there is no funding regarding the publication of this article.
Conflict of Interest
The authors indicated no potential conflict of interest.
Informed Consent
The patient’s guardians signed informed consent to publish this case report.
Author Contributions
KO and TS wrote the paper. SI, HS, YM, NS, TS, and MM reviewed the paper and gave conceptual advice. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
B19: human parvovirus B19; KD: Kawasaki disease; EI: erythema infectiosum; SLE: systemic lupus erythematosus; IC: immune complex; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; anti-dsDNA Ab: anti-double-stranded DNA antibodies; Ig: immunoglobulin
| References | ▴Top |
- Janovitz T, Wong S, Young NS, Oliveira T, Falck-Pedersen E. Parvovirus B19 integration into human CD36+ erythroid progenitor cells. Virology. 2017;511:40-48.
doi pubmed - Watanabe Y, Inoue Y, Takatani T, Arai H, Yasuda T. Self-limited lupus-like presentation of human parvovirus B19 infection in a 1-year-old girl. Pediatr Int. 2009;51(3):411-412.
doi pubmed - Moore TL, Bandlamudi R, Alam SM, Nesher G. Parvovirus infection mimicking systemic lupus erythematosus in a pediatric population. Semin Arthritis Rheum. 1999;28(5):314-318.
doi - Nigro G, Zerbini M, Krzysztofiak A, Gentilomi G, Porcaro MA, Mango T, Musiani M. Active or recent parvovirus B19 infection in children with Kawasaki disease. Lancet. 1994;343(8908):1260-1261.
doi - Holm JM, Hansen LK, Oxhoj H. Kawasaki disease associated with parvovirus B19 infection. Eur J Pediatr. 1995;154(8):633-634.
doi pubmed - Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733.
doi pubmed - Musiani M, Manaresi E, Gallinella G, Cricca M, Zerbini M. Recurrent erythema in patients with long-term parvovirus B19 infection. Clin Infect Dis. 2005;40(12):e117-119.
doi pubmed - Brown KE, Young NS, Alving BM, Barbosa LH. Parvovirus B19: implications for transfusion medicine. Summary of a workshop. Transfusion. 2001;41(1):130-135.
doi pubmed - Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15(3):485-505.
doi pubmed - Waldman M, Kopp JB. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol. 2007;2:47-56.
doi pubmed - Aslanidis S, Pyrpasopoulou A, Kontotasios K, Doumas S, Zamboulis C. Parvovirus B19 infection and systemic lupus erythematosus: Activation of an aberrant pathway? Eur J Intern Med. 2008;19(5):314-318.
doi pubmed - Seve P, Ferry T, Koenig M, Cathebras P, Rousset H, Broussolle C. Lupus-like presentation of parvovirus B19 infection. Semin Arthritis Rheum. 2005;34(4):642-648.
doi pubmed - Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341(27):2068-2074.
doi pubmed - Gasser S, Raulet D. The DNA damage response, immunity and cancer. Semin Cancer Biol. 2006;16(5):344-347.
doi pubmed - Kuo HC, Yang KD, Chang WC, Ger LP, Hsieh KS. Kawasaki disease: an update on diagnosis and treatment. Pediatr Neonatol. 2012;53(1):4-11.
doi pubmed - Knosel T, Meisel H, Borgmann A, Riebel T, Krenn V, Schewe C, Petersen I. Parvovirus B19 infection associated with unilateral cervical lymphadenopathy, apoptotic sinus histiocytosis, and prolonged fatigue. J Clin Pathol. 2005;58(8):872-875.
doi pubmed - Nigro G, Pisano P, Krzysztofiak A. Recurrent Kawasaki disease associated with co-infection with parvovirus B19 and HIV-1. AIDS. 1993;7(2):288-290.
doi pubmed - Yoto Y, Kudoh T, Haseyama K, Suzuki N, Chiba S, Matsunaga Y. Human parvovirus B19 infection in Kawasaki disease. Lancet. 1994;344(8914):58-59.
doi - Chua PK, Nerurkar VR, Yu Q, Woodward CL, Melish ME, Yanagihara R. Lack of association between Kawasaki syndrome and infection with parvovirus B19, human herpesvirus 8, TT virus, GB virus C/hepatitis G virus or Chlamydia pneumoniae. Pediatr Infect Dis J. 2000;19(5):477-479.
doi pubmed - Magro CM, Dawood MR, Crowson AN. The cutaneous manifestations of human parvovirus B19 infection. Hum Pathol. 2000;31(4):488-497.
doi pubmed - Takahashi M, Ito M, Sakamoto F, Shimizu N, Furukawa T, Takahashi M, Matsunaga Y. Human parvovirus B19 infection: immunohistochemical and electron microscopic studies of skin lesions. J Cutan Pathol. 1995;22(2):168-172.
doi pubmed - Mitchell LA. Parvovirus B19 nonstructural (NS1) protein as a transactivator of interleukin-6 synthesis: common pathway in inflammatory sequelae of human parvovirus infections? J Med Virol. 2002;67(2):267-274.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


