| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 3, March 2022, pages 140-144
Mitigating Gait Decline in a Woman With Parkinson’s Disease: A Case Report
Eric Chun-Pu Chua, c , Arnold Yu-Lok Wongb
aNew York Chiropractic and Physiotherapy Centre, Hong Kong, China
bDepartment of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hong Kong, China
cCorresponding Author: Eric Chun-Pu Chu, New York Chiropractic and Physiotherapy Centre, Hong Kong, China
Manuscript submitted November 21, 2021, accepted January 5, 2022, published online March 5, 2022
Short title: Mitigating Parkinsonian Gait Impairment
doi: https://doi.org/10.14740/jmc3856
| Abstract | ▴Top |
Levodopa therapy is the standard pharmacological treatment for Parkinson’s disease (PD). However, after an initial period of significant benefit, the effects of levodopa begin to wear off. This results in a reduction in the effect duration and the development of motor complications. We describe the case of a 69-year-old woman presented with a 3-year history of lower back pain and progressive left leg weakness. One year prior to referral for neurological assessment, the patient first noted progressive leg weakness and insufficient strength to rise from a chair. The diagnosis of PD was made after excluding potential neurological disorders. The patient was initially started on oral levodopa, which improved her motor symptoms considerably during the first year. However, dose adjustment and combined pharmacological strategies failed to sufficiently control motor symptoms during the subsequent year. The patient experienced declines in gait ability, clumsiness in the left limbs, and difficulty in performing housework. The patient then sought chiropractic attention. Gait rehabilitation was the major goal in the treatment program for this patient, with the impression of motor complications of PD. The intervention consisted of spinal manipulation, intermittent motorized traction of the lumbar segments, and gait training programs. Following 3 months of the intervention, the patient demonstrated increased muscle strength and improved gait characteristics, as depicted by a gait cyclogram and vertical ground reaction force graphing. The current report illustrates that a multicomponent chiropractic approach may be used as an additional measure to mitigate gait decline in PD patients.
Keywords: Exercise rehabilitation; Gait cyclogram; Gait decline; Parkinson’s disease; Spinal manipulation
| Introduction | ▴Top |
Dopamine replacement therapy using the precursor levodopa (L-DOPA) is the standard treatment for Parkinson’s disease (PD). Over time, following a continued degeneration of dopaminergic neurons [1], the brain begins to rely on the most recent dose of medication. With both weaning and lack of response, dopaminergic therapy demonstrates reduced effect when it has been used for a certain duration [2]. Gait problems, such as freezing of gait, reduced balance, and postural control, become more evident. Continuous stimulation (using device-aided therapies or deep brain stimulation) may help in controlling motor fluctuations, dyskinesias, and cardinal motor symptoms [3]. However, each advanced therapy has both advantages and disadvantages, and not all patients are suitable for these therapies. Gait problems remain an issue, thereby, warranting additional therapeutic interventions.
The current study serves as an example of mitigating gait impairment and restoring postural control in patients with PD. Some musculoskeletal problems can be treated with different types of manual therapy to improve muscle strength, balance, and joint mobility. Spinal manipulation and exercise-based interventions are examples of alternative options to address the limitations of dopaminergic therapy. Gait variability, the stride-to-stride fluctuations in walking, is a quantifiable feature of gait that alters with disease, in addition to the effects of therapeutic interventions and rehabilitation [4]. Here, we review contemporary insights into gait improvement following rehabilitation interventions.
| Case Report | ▴Top |
Investigations
A 69-year-old female presented with a 3-year history of progressive left leg weakness and unsteadiness of gait. Three years prior to our diagnosis, the patient presented with weakness of the left leg, slowing during walking, insufficient strength to rise from a chair, and lower back pain during a visit to her family physician. The patient was subsequently diagnosed with lumbar spondylosis. With no clear improvement following medication and physiotherapy for motor symptoms for 12 months, the patient was then referred to a neurologist. She was diagnosed with PD according to the Movement Disorder Society clinical diagnostic criteria [5]. Oral levodopa treatment was prescribed, and the patient responded well during the first year.
Following 1 year of treatment, the patient began to feel that the effects of her medication were increasingly short-lived. Leg weakness, bradykinesia, and postural instability progressed over the next 12 months, despite repeated dose adjustments and combined pharmacological strategies. Aggravation of motor impairment progressed, which affected the left upper limb. The patient experienced difficulties in performing housework and became increasingly frustrated by her motor symptoms. As such, she sought chiropractic attention for her back pain and motor symptoms.
Diagnosis
At presentation, the patient exhibited slowing and walked cautiously with an irregular wide-based gait. Resting tremor was observed in her left hand and left leg. The patient’s self-reported peak pain intensity of her back pain was 4 out of 10 on an 11-point numeric pain rating scale. Physical examination showed stiffness in the lower back, tenderness to palpation over the lumbosacral spine, reduced muscle strength of the left hip flexors and knee extensors, and hypertonicity of the left soleus and hamstring muscles. The patient’s sensorium and cognition were intact. Motor strength measurements with a handheld dynamometer were graded at 4/5 of the left hand and 3/5 of the left leg. A walking trajectory (butterfly cyclogram) [6] depicted irregular stride length, great variations in stepping location, and a wider base of support (Fig. 1a).
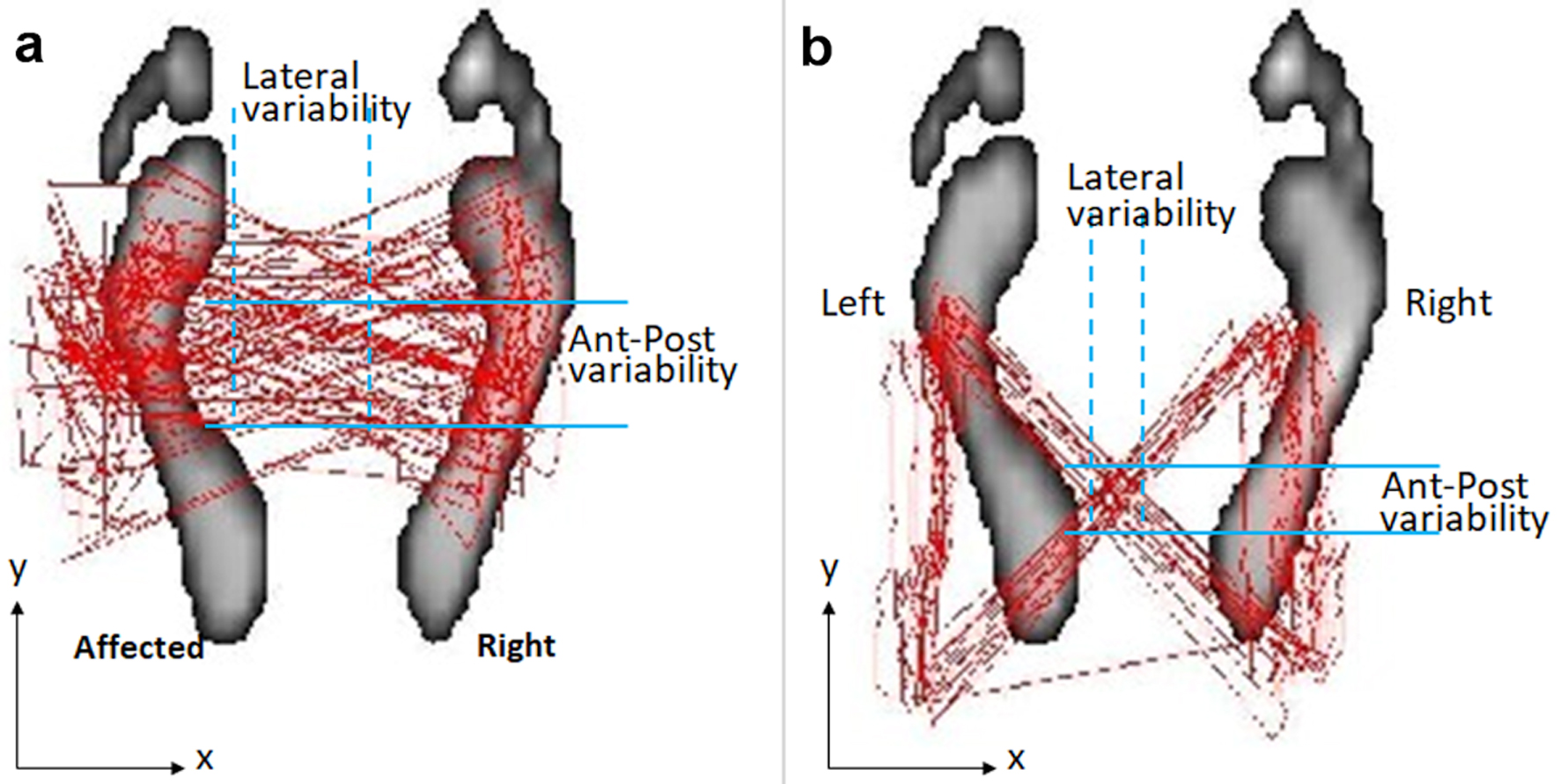 Click for large image | Figure 1. Walking cyclogram depicting notable improvement in gait characteristics after treatment. The traces (red butterfly plots) represent the trajectory of center of foot pressure on the instrumented treadmill. (a) Initially, the cyclogram showed a distorted butterfly wing feature, with a wider based gait, and great variability of stride and foot placement accuracy (blue intersected area). The disturbance was more pronounced on the left side. (b) After 3 months of intervention, the repeated cyclogram showed a more accurate foot stride, regular heel strike and symmetric and rhythmic center-of-pressure crossover between strides. |
Based on medical history and results of the test and measures, it was established that the patient had symptoms of PD motor complications. The primary medical International Classification of Diseases, Tenth Edition (ICD-10) codes were G20 (Parkinson’s disease) and M47.816 (spondylosis without myelopathy or radiculopathy, lumbar region), and the primary physical therapy ICD-10 code was R26.9 (unspecified abnormalities of gait and mobility).
Treatment
The treatment plan was aimed at maintaining the patient’s function whilst increasing muscle strength, balance, and gait ability, all of which would give potentially the patient the confidence to carry out tasks. Chiropractic intervention consisted of lumbar spine manipulation and intermittent motorized lumbar traction to release intersegmental restriction. Additionally, therapeutic ultrasound and massage to provide muscle flexibility, along with biceps curl balance and balance board training to retrieve balance were used. The frequency of treatment was twice weekly. Furthermore, home exercise recommendations included heel toe raises, seated marching, weight shifting, side-lying leg raise, and back bridging, in an attempt to improve walking ability.
Follow-up and outcomes
Following 3 months of the intervention the patient showed improvements in back pain and motor symptoms. This was shown by a decrease in pain scores (from 4 to 0 on an 11-point numeric rating scale) and an increase in dynamometer strength readings (an increase to 4+/5 in left hand and to 4/5 in left leg muscle strength). Additionally, an improvement in gait characteristics was shown by a more accurate foot stride, a regular heel strike and a symmetric and rhythmic center-of-pressure crossover between strides as depicted by a walking cyclogram (Fig. 1b). Furthermore, an improvement in foot pressure and a regular stance phase were recorded as vertical ground reaction force (GRF) graphing [7] (Fig. 2). The patient has been followed up for 12 months, and she continued her normal daily activities. No treatment-related adverse events were reported.
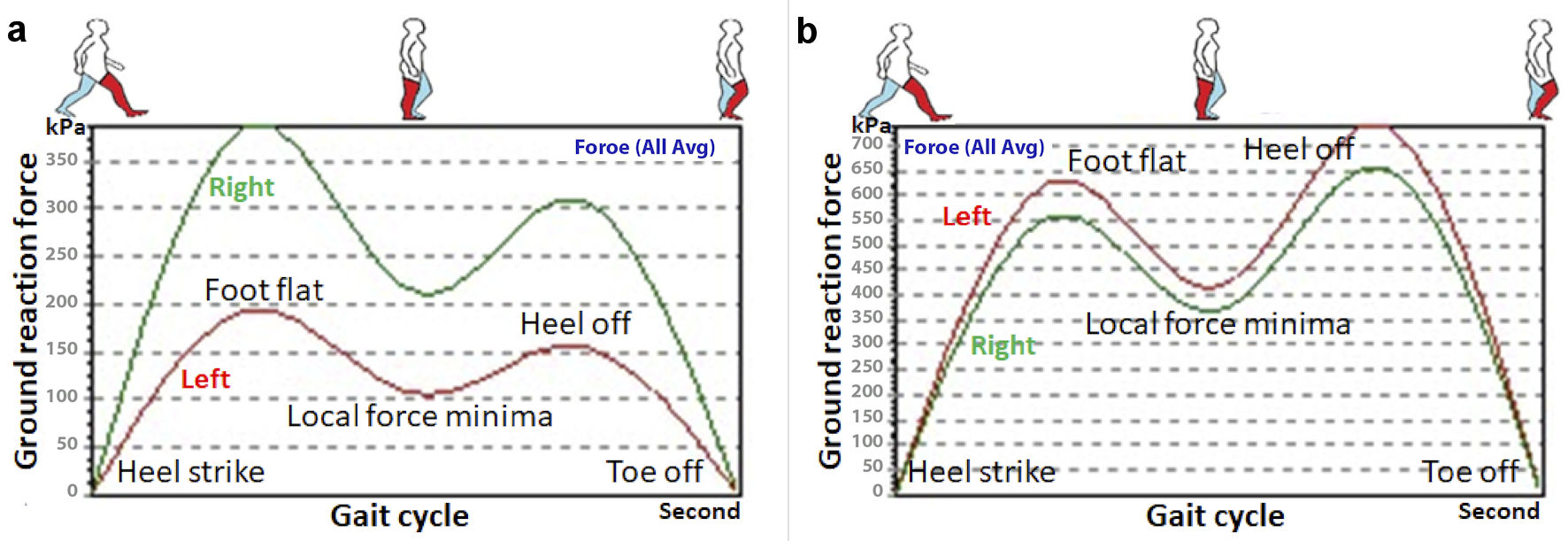 Click for large image | Figure 2. Graph comparing the gait pattern on vertical ground reaction force (GRF). (a) At initial presentation, the patient demonstrated a marked decrease in applied force of the left foot in the stance phase of the gait cycle, as compared to the right foot. (b) The follow-up vertical GRF graph recorded symmetric foot pressure and regular stance phase. It could be noted that the GRF peaks were markedly increased after treatment. The data represent the average vertical force (in kPa) of multiple stride cycles recorded by pressure foot sensors as the patient walked for 2 min on the instrumented treadmill. |
| Discussion | ▴Top |
The mechanism by which a patient’s response to levodopa changes during long-term therapy is not fully understood. When the motor signs of PD emerge, approximately 20-40% of dopaminergic neurons may be remaining [8]. Presumably, the ingested levodopa is taken up by the remaining dopaminergic neurons and is converts to dopamine, which is stored and slowly released into the synaptic cleft. However, as the degeneration of dopamine neurons progresses, this conversion, storage, and release mechanism is compromised [8]. Plasma levels of levodopa will show extreme variations, and the response to levodopa become unpredictable, resulting in the appearance of motor fluctuations [1]. When gait decline cannot be effectively solved by pharmacological means, additional interventions to mitigate gait impairment and its progression are warranted [2]. However, exercise rehabilitation has become a promising adjunct treatment for PD [9].
Patients with PD display profound reductions in isokinetic muscle strength, which causes functional difficulties. The deficit in central activation [10] and the rate of force generation of muscles [11, 12] during repeated strengthening are responsible for muscle weakness. Increasing evidence suggests that exercise and motor training improves force-generating capacity, neuromuscular function, and functional performance [11, 13]. Spinal manipulation therapy, broadly speaking, is primarily used to treat musculoskeletal issues, with emphasis on a range of strategies to mobilize restricted structures and relieve neural compromise, and maximize the functions of contracted joints and affected muscles [4]. The positive changes observed after spinal manipulation and exercise-based training may be unrelated to treatment specificity but a systemic effect of functional changes in a biomechanical interdependence [14]. It is speculated that pain relief following manipulative remedies may improve disc diffusion, neural proprioception of muscles, motor functions, and postural balance [4, 14].
Several factors may contribute to the optimization of gait ability in patients with PD. Gait impairment is the outcome of a complex interaction of disease progression, aging changes, compensatory mechanisms, and eventual secondary deconditioning due to restricted mobility. Speculatively, exercise-based interventions aimed at increasing muscle strength and activity may, at least partially, target age-related changes (sarcopenia, physical inactivity, osteoarthritis, etc.), which may positively impact PD gait [2]. Although the underlying mechanisms are multifactorial, sometimes all it takes is pain relief and gait improvement to increase a patient’s motivation to continue, which may have led to even better outcomes. With regard to this case, by facilitating muscle strength and spinal excitability, chiropractic intervention itself might have substantially contributed to neuromuscular and mobility improvement in parkinsonian gait [4].
Researchers [15, 16] have observed that peripheral blood levels of brain-derived neurotrophic factor (BDNF) increased significantly after a single bout of aerobic or strength exercise, as well as after a training session. As a member of the neurotrophin family of growth factors, BDNF has a significant effect in promoting neurogenesis, synaptic plasticity, neuronal survival, and reducing inflammation. BDNF acts as a protective agent for dopaminergic neurons and is a promoter of neuroplasticity [17]. Neuroplasticity refers to the capacity of the nervous system to modify itself in response to experience and injury [18]. Therefore, BDNF facilitates exercise-based recovery [19].
Individuals with gait dysfunction demonstrate increased stride-to-stride fluctuations, which are known as gait variability. PD patients with axial disability typically adjust their movement pattern to move slowly and safely to avoid falls or injuries [4]. A more variable step width may compensate for balance difficulties [2]. Measurements of gait cadence, symmetry, stride variables, and foot placement characteristics can be useful markers of disease progression and response to therapy. Instrumented treadmill analysis systems can be implemented, which can measure stepping paradigms during ambulation. The overlaid graphical display of dynamic foot placement [6] is an intuitive way to quantify subtle changes in walking difficulty during therapeutic interventions (Fig. 1).
This report provides novel evidence that patients with PD can become significantly more functional in a relatively short amount of time after spinal manipulation with motor training, demonstrating a decreased pain score, increased muscle strength measurement, and improved gait characteristics depicted on gait cyclogram and vertical GRF graphing (Figs. 1, 2). A focus on movement strategies may therefore lead to the development of useful therapies. While it is often difficult to generalize results from a single case to a larger population, the actual duration of sustaining improved outcomes following a course of manipulative intervention remains to be determined.
Conclusions
This case report provides new evidence and highlights the value of chiropractic intervention in making neuromuscular and mobility improvement in parkinsonian gait, especially when the problem cannot be effectively solved by pharmacological means. It contributes significantly to the existing practice in the treatment for PD.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient for case publication.
Author Contributions
Each author has individually been involved in and has made substantial contributions to conception and design, acquisition of data, analysis, interpretation of data, drafting and editing the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BDNF: brain-derived neurotrophic factor; GRF: ground reaction force; ICD: International Classification of Diseases; PD: Parkinson’s disease
| References | ▴Top |
- Aradi SD, Hauser RA. Medical management and prevention of motor complications in Parkinson's disease. Neurotherapeutics. 2020;17(4):1339-1365.
doi pubmed - Wilson J, Alcock L, Yarnall AJ, Lord S, Lawson RA, Morris R, Taylor JP, et al. Gait progression over 6 years in Parkinson's disease: effects of age, medication, and pathology. Front Aging Neurosci. 2020;12:577435.
doi pubmed - di Biase L, Di Santo A, Caminiti ML, De Liso A, Shah SA, Ricci L, Di Lazzaro V. Gait analysis in Parkinson's disease: An overview of the most accurate markers for diagnosis and symptoms monitoring. Sensors (Basel). 2020;20(12):3529.
doi pubmed - Chu ECP, Wong AYL, Lee LYK. Chiropractic care for low back pain, gait and posture in a patient with Parkinson's disease: a case report and brief review. AME Case Rep. 2021;5:34.
doi pubmed - Postuma RB, Poewe W, Litvan I, Lewis S, Lang AE, Halliday G, Goetz CG, et al. Validation of the MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2018;33(10):1601-1608.
doi pubmed - Shin C, Ahn TB. Asymmetric dynamic center-of-pressure in Parkinson's disease. J Neurol Sci. 2020;408:116559.
doi pubmed - Kim HD, Kim JG, Jeon DM, Shin MH, Han N, Eom MJ, Jo GY. Analysis of vertical ground reaction force variables using foot scans in hemiplegic patients. Ann Rehabil Med. 2015;39(3):409-415.
doi pubmed - Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62(1):1-8.
doi pubmed - Li X, He J, Yun J, Qin H. Lower limb resistance training in individuals with Parkinson's disease: an updated systematic review and meta-analysis of randomized controlled trials. Front Neurol. 2020;11:591605.
doi pubmed - Stevens-Lapsley J, Kluger BM, Schenkman M. Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson disease. Neurorehabil Neural Repair. 2012;26(5):533-541.
doi pubmed - Helgerud J, Thomsen SN, Hoff J, Strandbraten A, Leivseth G, Unhjem R, Wang E. Maximal strength training in patients with Parkinson's disease: impact on efferent neural drive, force-generating capacity, and functional performance. J Appl Physiol (1985). 2020;129(4):683-690.
doi pubmed - Hammond KG, Magrini MA, Siedlik JA, Bickel CS, Bamman MM. Influence of muscle fatigue on contractile twitch characteristics in persons with parkinson's disease and older adults: A pilot study. Clin Park Relat Disord. 2021;5:100103.
doi pubmed - Meng Z, Xo Z, Li B, Zheng Y, Li L, Du Fei LT, Yan Z, et al. Meta-analysis: The effect of muscle strength training on walking ability of patients with Parkinson's disease. Rehabil Sci. 2021;6(1):1-9.
doi - Nim CG, Downie A, O'Neill S, Kawchuk GN, Perle SM, Leboeuf-Yde C. The importance of selecting the correct site to apply spinal manipulation when treating spinal pain: Myth or reality? A systematic review. Sci Rep. 2021;11(1):23415.
doi pubmed - Marinus N, Hansen D, Feys P, Meesen R, Timmermans A, Spildooren J. The impact of different types of exercise training on peripheral blood brain-derived neurotrophic factor concentrations in older adults: a meta-analysis. Sports Med. 2019;49(10):1529-1546.
doi pubmed - Arazi H, Babaei P, Moghimi M, Asadi A. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021;21(1):50.
doi pubmed - Dominguez-Sanchez MA, Bustos-Cruz RH, Velasco-Orjuela GP, Quintero AP, Tordecilla-Sanders A, Correa-Bautista JE, Triana-Reina HR, et al. Acute effects of high intensity, resistance, or combined protocol on the increase of level of neurotrophic factors in physically inactive overweight adults: the BrainFit study. Front Physiol. 2018;9:741.
doi pubmed - von Bernhardi R, Bernhardi LE, Eugenin J. What Is Neural Plasticity? Adv Exp Med Biol. 2017;1015:1-15.
doi pubmed - Bilchak JN, Caron G, Cote MP. Exercise-induced plasticity in signaling pathways involved in motor recovery after spinal cord injury. Int J Mol Sci. 2021;22(9):4858.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


