| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 5, May 2022, pages 202-206
Pseudoaneurysm Formation After “Preclose”-Assisted Impella Insertion in a Patient With Cardiogenic Shock
Daniel Asemotaa, c, Zain Kassama, Christian Votob, Aditya Manglaa, David Covena, Zoran Lasica
aDepartment of Cardiology, Jamaica Hospital Medical Center, Lenox Hill Hospital, Northwell Health, New York, NY, USA
bDepartment of Medicine, Jamaica Hospital Medical Center, Lenox Hill Hospital, Northwell Health, New York, NY, USA
cCorresponding Author: Daniel Asemota, Department of Cardiology, Jamaica Hospital Medical Center, Lenox Hill Hospital, Northwell Health, New York, NY, USA
Manuscript submitted November 4, 2021, accepted December 8, 2021, published online April 23, 2022
Short title: Impella Insertion Complicated by PA Formation
doi: https://doi.org/10.14740/jmc3841
| Abstract | ▴Top |
The use of mechanical support devices such as the Impella CP (Abiomed, Danvers, MA) is a growing form of treatment for patients with cardiogenic shock (CS). Despite the increase in usage, there remains a dearth in literature regarding potential complications. Vascular complications such as pseudoaneurysms (PAs) are rare but important potential complications that can occur with use of the Impella. We present Impella-assisted percutaneous coronary intervention (PCI) in a patient with CS, “Preclosed” with the Perclose ProGlide (Abbott, Plymouth, MN) device complicated by development of a PA. A 62-year-old male patient with a history of diabetes and hypertension presented to our emergency room (ER) with chest pain and electrocardiogram (ECG) findings consistent with an acute anterior wall ST-elevation myocardial infarction (STEMI). This was further complicated by refractory CS. The patient was urgently taken to the cardiac catherization laboratory. After exchange of sequential dilators, a single Perclose device was used prior to the insertion of the Impella sheath. The patient then underwent a successful Impella-assisted PCI of his left anterior descending artery. Upon stabilization of hemodynamics, the patient was taken to the catheterization laboratory for Impella removal. After removal of Impella, imaging detected extravasation of contrast, without development of hematoma, later confirmed to be a PA via computed tomography (CT) scans and ultrasound Doppler imaging. The PA was successfully managed with injection of thrombin. The PA was likely caused by shearing forces of the dilators, the 14-F Impella sheath and foot of the device. We propose deploying the Perclose device earlier in the process of dilating the access site to avoid such complication. This is one of the first case reports that detail the occurrence and management of a PA with Impella insertion.
Keywords: Pseudoaneurysm; Impella complication; Cardiogenic shock
| Introduction | ▴Top |
Cardiogenic shock (CS) is the leading cause of in-hospital mortality in ST-elevation myocardial infarction (STEMI). The use of temporary mechanical circulatory support (MCS) during high-risk percutaneous coronary intervention (PCI), such as intra-aortic balloon pump (IABP), Impella, and extracorporeal membrane oxygenation (ECMO) to increase stroke volume and/or reduce end-diastolic left ventricle pressure is an attractive option that may improve outcomes [1-3]. Of the MCS devices, the Impella is being increasingly used as an alternative to the IABP and ECMO for hemodynamic support in patients with CS and high-risk PCI due to its superior ability to augment cardiac output (CO) and reduce shock severity parameters, although it has yet to show significant effect on reducing 30-day mortality [1, 4].
Impella is a percutaneous system which consists of an intracardiac micro-axial rotary pump used for high-risk PCIs. Large-bore arterial access is required for placement of Impella devices. Typically, a transfemoral approach is utilized with retrograde cannulation of the common femoral artery (CFA) under ultrasound and fluoroscopic guidance and subsequent femoral angiogram through a small-sized sheath [5]. Once the femoral artery is determined to be adequate in size and free of significant disease, the access site can be used for Impella insertion. Closure of the arteriotomy site can be challenging with long manual compression. As an alternative, percutaneous suture closure can be used reducing patient discomfort from prolonged compression [6]. The “Preclose” technique, where one (or two) Perclose suture device is used instead of manual compression, represents a useful method to achieve hemostasis and may alleviate patient discomfort and allow for quicker time-to-ambulation and discharge [6].
Despite its clear benefits, there are several possible complications associated with the use of the Impella device. Iatrogenic aortic and mitral valve injuries are rare but potential complications following implantation of the Impella device. Additionally, vascular access complications such as hematoma or pseudoaneurysm (PA), limb ischemia, distal thrombus formation, stroke, and major bleeding have all been reported in literature [3, 4, 7-9]. In this case report, we examine the occurrence of an iatrogenic PA in the setting of Impella-assisted high-risk PCI in a patient with an acute anterior wall STEMI complicated by refractory CS. During this procedure, a Perclose was deployed using the “Preclose” technique before Impella insertion.
| Case Report | ▴Top |
Investigations
A 62-year-old diabetic and hypertensive male patient presented to our emergency room (ER) with chest pain; electrocardiogram (ECG) revealed an anterior wall STEMI. He was administered 325 mg of aspirin, 180 mg of brilinta, and 5,000 units of heparin, and urgently taken to the catherization laboratory. Coronary angiography revealed a 100% occlusion of the proximal left anterior descending artery, deemed to be the culprit lesion. Ventriculography was suggestive of severely reduced left ventricular ejection fraction of 20%; hemodynamic parameters were consistent with refractory cardiogenic shock.
Diagnosis
After left and right heart catheterization, prior to PCI, an Impella CP was inserted via the right common femoral artery (RCFA); a single Perclose device was used with the “Preclose” technique: the Perclose device was deployed after sequential dilatation and placement of the short Impella sheath. Between dilators and sheath exchanges, hemostasis was maintained via manual compression. The patient underwent successful Impella-assisted PCI of the culprit lesion with excellent angiographic results and then transferred to the intensive care unit (ICU) for further monitoring. The following day, the patient returned to the catherization laboratory for Impella removal. The Impella CP was removed, and hemostasis was achieved using the Perclose closure device and short manual compression. The patient however endorsed discomfort in the right groin after the hemostasis, without evidence of hematoma development or change in peripheral pulses. Repeat femoral angiogram in antegrade fashion from CFA contralateral access revealed extravasation of contrast at the level of RCFA (Fig. 1a). An additional 10 min of manual compression and 5 min of tamponade using a Mustang 8.0 × 40.0 mm balloon (Boston Scientific, Marlborough, MA) to achieve hemostasis was ineffective (Fig. 1b). While there was still no evidence of hematoma formation, emergent CT imaging revealed a PA arising from the RCFA (Fig. 2), subsequently confirmed by duplex ultrasound (Fig. 3a). At time of PA discovery, notable lab values were: serum hemoglobin 11.6 g/dL, serum platelets 215 × 103/µL, international normalized ratio 0.9 s, partial thromboplastin time 24.2 s.
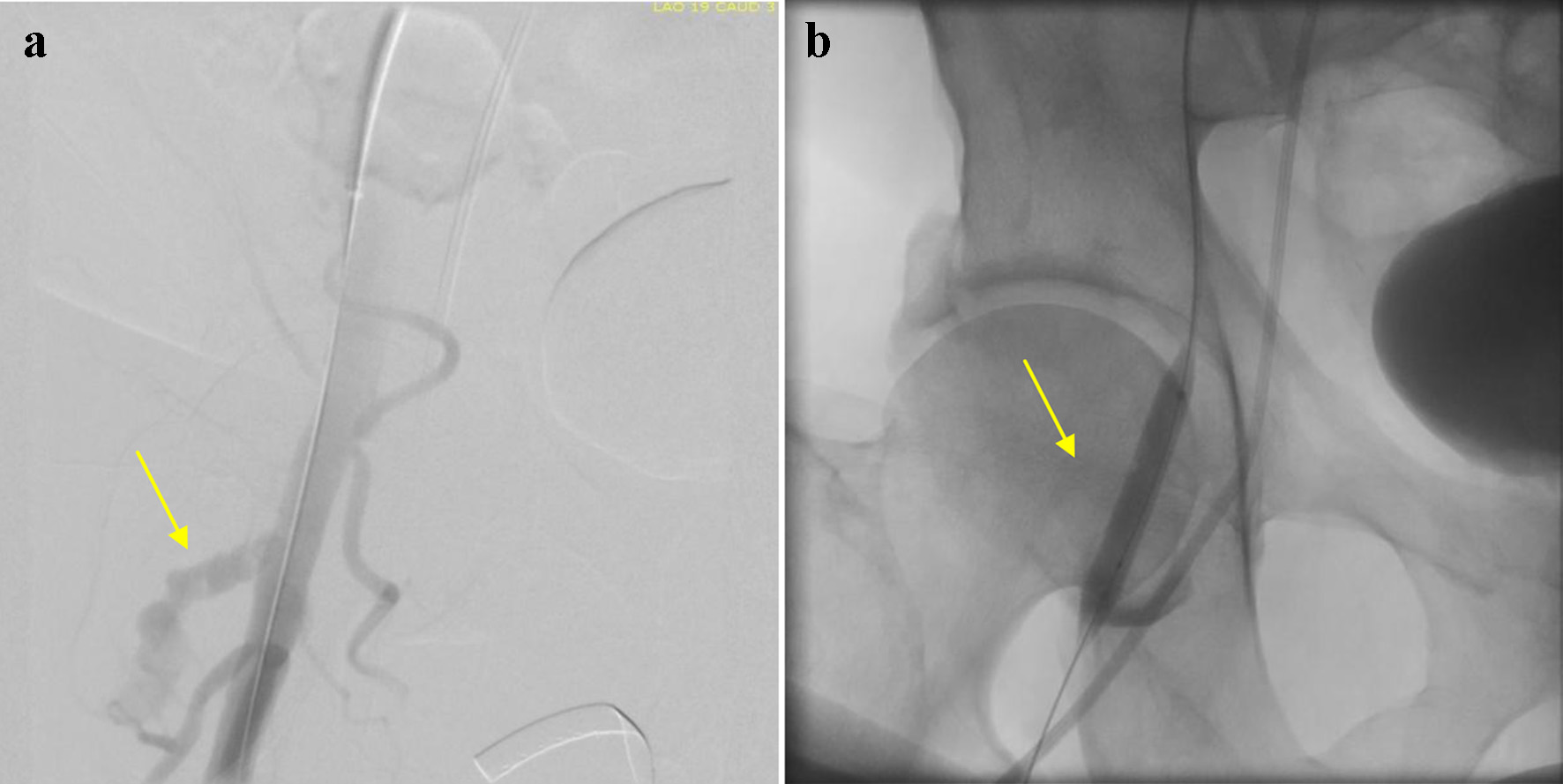 Click for large image | Figure 1. (a) Femoral angiogram displaying extravasation of contrast at the level of right common femoral artery (arrow). (b) Attempt to occlude common femoral artery via balloon tamponade method (arrow). |
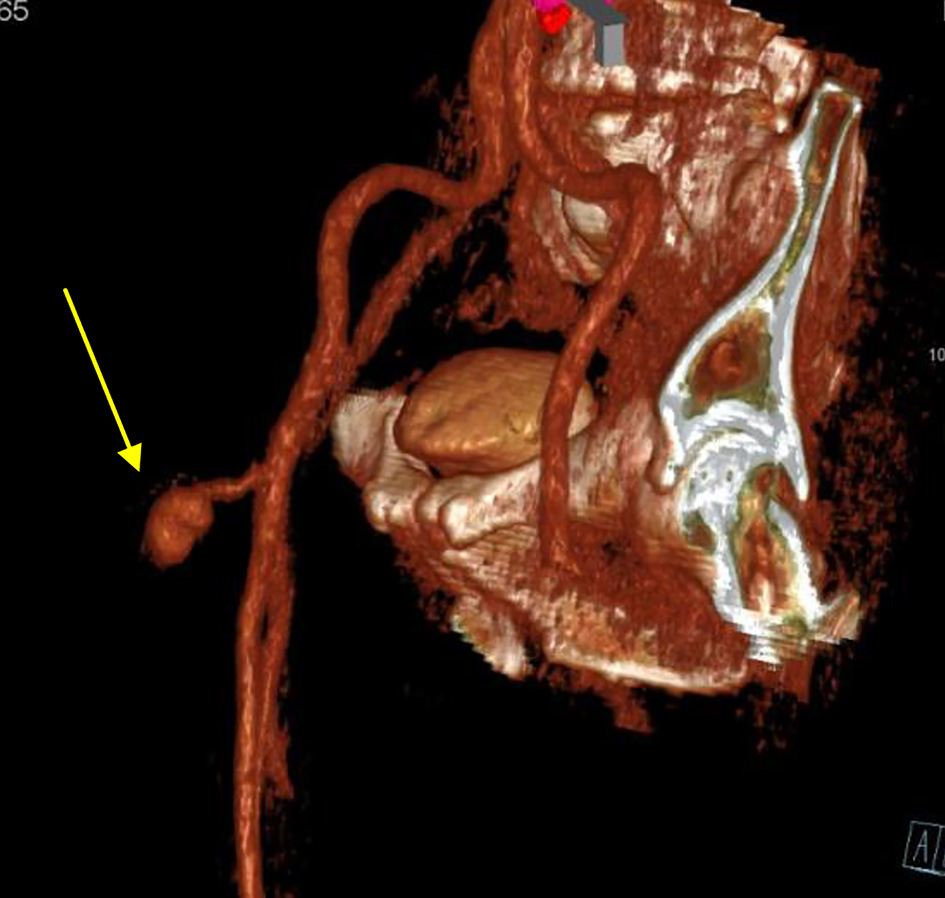 Click for large image | Figure 2. Computed tomography angiogram on delayed venous phase and arterial 3D reconstruction reveals a pseudoaneurysm arising from the common femoral artery (arrow). |
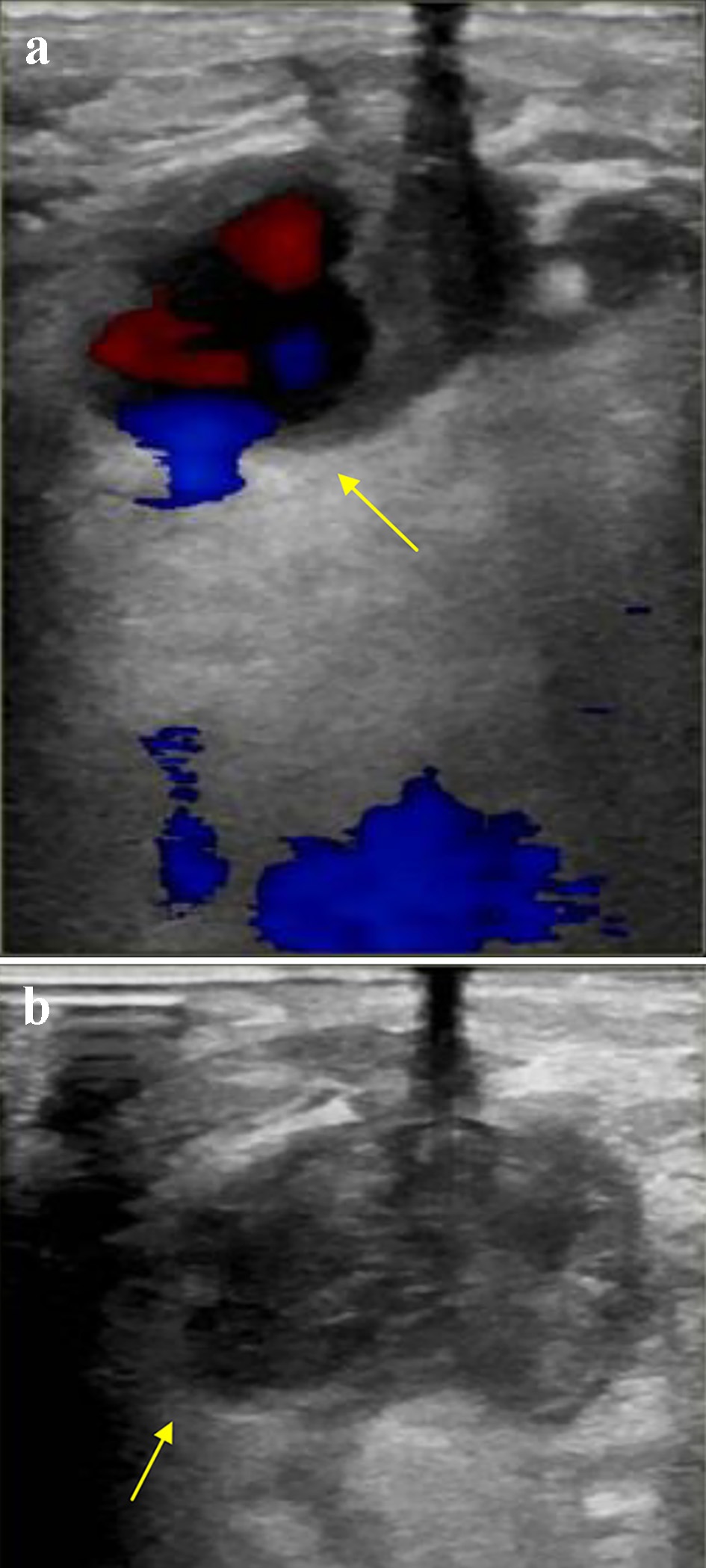 Click for large image | Figure 3. (a) Color flow on arterial duplex ultrasound of the right common femoral artery demonstrating (a) “To-and-Fro” flow through a narrow neck, confirming a pseudoaneurysm (arrow), and (b) no flow within the aneurysmal sac, confirming successful thrombosis of the pseudoaneurysm (arrow). |
Treatment
Vascular surgery was consulted and deemed the PA was amenable for ultrasound-guided intervention given the presence of a narrow neck connecting the CFA to the aneurysmal sac. The PA was treated successfully with injection of 1,000 units of thrombin. Figure 3b reveals successful thrombosis evidence by lack of flow within the aneurysmal sac.
Follow-up and outcomes
Following resolution of the PA, patient’s pain resolved, and he was discharged home in a stable condition.
| Discussion | ▴Top |
Placement and use of the Impella device bear potential risks. Risk factors for worse outcomes with Impella include older patients and female gender due to the higher likelihood of severe peripheral vascular disease and smaller arterial anatomy, respectively [3, 4]. Additionally, diabetes mellitus and chronic kidney disease are associated with a negative impact on outcome. According to several review articles and meta-analyses, the most common complications reported are major bleeding requiring blood product transfusion (15-20%), vascular access complications (about 15%), infection (about 13%), hemolysis (about 10%), vascular complications with a need of surgical repair (about 10%), limb ischemia (about 3-7%), stroke (about 4%) and bleeding requiring surgical intervention (about 2%) [3, 4, 9].
The urgency at which a particular patient presents may also preclude a dedicated, meticulous access approach compared to patients undergoing an elective or urgent procedure, as an optimal sheath to femoral artery ratio is fundamental to reducing risk of limb ischemia. While there was no significant association between the use of a closure device and the development of vascular complications, Abaunza et al reported that none of the patients who underwent an elective high-risk cardiac procedure had any vascular complications, while 21% patients undergoing urgent PCI and 40% who underwent emergent PCI experienced vascular complications [7].
Furthermore, the use of vasopressors in patients with CS results in vasoconstriction and may increase the risk for vascular complications [3].
For every 1-mm decrease in vessel size, there was a 35% increase in the odds of having a vascular complication (odds ratio, 1.35; 95% confidence interval, 0.94 - 2.02) [7].
Contraindications (relative and absolute) to its use therefore exist, such as severe peripheral vascular disease due to the unacceptably high risk of lower limb ischemia with femoral access [4]. Another study examining 61 Impella devices found the rate of major vascular complications, as defined by the Valve Academic Research Consortium-2 criteria, to be 8% while the rate of minor vascular complications to be 36% [10]. Major vascular complications utilizing these criteria are defined by access-related vascular injury (including PA) leading to death, life-threatening bleeding, and distal embolization (non-cerebral) from a vascular source requiring surgery, resulting in amputation, or irreversible end-organ damage among others.
Intervention of a PA is dictated by the size and hemodynamic stability of the patient. Uncomplicated PAs such as the one described in this case can be successfully managed by injection of thrombin [11, 12]. Other methods such as ultrasound-guided compression and endovascular stenting have been described as well, with endovascular stenting reserved for cases where ultrasound thrombin injection was unsuccessful or technically difficult [13]. Open repair of a PA is reserved for patients who are hemodynamically unstable or have features that would result in unsuccessful ultrasound-guided thrombosis such as a PA with a wide neck [11, 13].
Our case illustrates the possibility of an iatrogenic PA formation, when using a Perclose device with the “Preclose” technique during Impella insertion. We hypothesize that the PA formation was likely caused by shearing forces of the dilators, the 14-F Impella sheath and foot of the device on the arterial wall, and traction toward the subcutaneous fat layer of the skin while applying manual pressure during the exchanges. This highlights the importance of deployment of the Perclose device after an 8-F or 10-F dilator (preferably 2), but before 12-F dilator or Impella sheath insertion. With bigger vessel wall opening, forceful manual bleeding control during exchange and deployment of Perclose could result in anatomical distortion and inadvertent pull of Perclose anchoring part toward the skin and subsegment PA formation.
Learning points
Properly timed deployment of the Perclose device in the “Preclose” fashion can prevent vascular complications such as PA formation.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
DA, ZK and CV performed research including data collection and wrote the paper. AM, DC and ZL designed research and provided expert clinical knowledge to revise critically.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Trpkov C, Gibson JD, Miller RJH, Grant ADM, Schnell G, Har BJ, Clarke B. Percutaneous left ventricular assist device in cardiogenic shock: a five-year single Canadian center initial experience. CJC Open. 2020;2(5):370-378.
doi pubmed - O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, Palacios I, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717-1727.
doi pubmed - Iannaccone M, Albani S, Giannini F, Colangelo S, Boccuzzi GG, Garbo R, Brilakis ES, et al. Short term outcomes of Impella in cardiogenic shock: A review and meta-analysis of observational studies. Int J Cardiol. 2021;324:44-51.
doi pubmed - Sef D, Kabir T, Lees NJ, Stock U. Valvular complications following the Impella device implantation. J Card Surg. 2021;36(3):1062-1066.
doi pubmed - Kelm M, Perings SM, Jax T, Lauer T, Schoebel FC, Heintzen MP, Perings C, et al. Incidence and clinical outcome of iatrogenic femoral arteriovenous fistulas: implications for risk stratification and treatment. J Am Coll Cardiol. 2002;40(2):291-297.
doi - Lata K, Kaki A, Grines C, Blank N, Elder M, Schreiber T. Pre-close technique of percutaneous closure for delayed hemostasis of large-bore femoral sheaths. J Interv Cardiol. 2018;31(4):504-510.
doi pubmed - Abaunza M, Kabbani LS, Nypaver T, Greenbaum A, Balraj P, Qureshi S, Alqarqaz MA, et al. Incidence and prognosis of vascular complications after percutaneous placement of left ventricular assist device. J Vasc Surg. 2015;62(2):417-423.
doi pubmed - Johannsen L, Mahabadi AA, Totzeck M, Krueger A, Janosi RA, Rassaf T, Al-Rashid F. Access site complications following Impella-supported high-risk percutaneous coronary interventions. Sci Rep. 2019;9(1):17844.
doi pubmed - Vargas KG, Jager B, Kaufmann CC, Biagioli A, Watremez S, Gatto F, Ozbek C, et al. Impella in cardiogenic shock following acute myocardial infarction: a systematic review and meta-analysis. Wien Klin Wochenschr. 2020;132(23-24):716-725.
doi pubmed - Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg. 2012;42(5):S45-60.
doi pubmed - Hood DB, Mattos MA, Douglas MG, Barkmeier LD, Hodgson KJ, Ramsey DE, Sumner DS. Determinants of success of color-flow duplex-guided compression repair of femoral pseudoaneurysms. Surgery. 1996;120(4):585-588; discussion 588-590.
doi - Cope C, Zeit R. Coagulation of aneurysms by direct percutaneous thrombin injection. AJR Am J Roentgenol. 1986;147(2):383-387.
doi pubmed - Luedde M, Krumsdorf U, Zehelein J, Ivandic B, Dengler T, Katus HA, Tiefenbacher C. Treatment of iatrogenic femoral pseudoaneurysm by ultrasound-guided compression therapy and thrombin injection. Angiology. 2007;58(4):435-439.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


