| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 2, Number 5, October 2011, pages 190-193
Erlotinib May be More Effective for Central Nervous Metastasis of Lung Adenocarcinoma Than Gefitinib Because of the Difference in the Clinical Regimes
Yumiko Imahashia, b, c, Naoko Shinmurab, Daijiro Nabeyab, Kyoko Inuib, Takashi Uejib, Mio Hayakawab, Kenichiro Otanib, Kyoko Yagyub, Takao Kamimorib, Hiroshi Fujiwarab
aOsaka City University Department of respiratory medicine, Osaka, Japan
bYodogawa Christian Hospital Department of respiratory medicine, Osaka, Japan
cCorresponding author: Yumiko Imahashi, Yodogawa Christian Hospital, department of respiratory medicine, 2-9-26, Awaji, Higashiyodogawa-ku, Osaka-City, Osaka 533-0032, Japan
Manuscript accepted for publication July 29, 2011
Short title: Erlotinib and Brain Metastasis of Lung Cancer
doi: https://doi.org/10.4021/jmc253w
| Abstract | ▴Top |
A 54-year-old woman with lung adenocarcinoma which has EGFR point mutation at exon 21 initially improved by administration of gefitinib. Lung lesions responded to gefitinib at 250 mg, but central nervous system lesions, such as carcinomatous meningitis and brain metastasis occurred. The therapy was changed from gefitinib to erlotinib at 150 mg, resulting in remarkable improvement of carcinomatous meningitis and brain metastasis improved dramatically. We hypothesized that the effective concentration of the clinical dose of erlotinib in the CSF is higher than that of gefitinib.
Keywords: Erlotinib; EGFR; Gefitinib; Carcinomatous meningitis; Clinical dose
| Introduction | ▴Top |
Carcinomatous meningitis develops in about 5% of patients with non-small cell lung cancer [1]. The median expected survival time without treatment is reported to be four to six weeks [2]. Treatment protocols include: radiation to the area containing the site of the tumor or the site of the symptoms; and intraventricular administration of chemotherapeutic drugs. However, even with treatments, the median expected survival time may be as short as two to six months [1]. We have encountered a case in which there was development of carcinomatous meningitis during gefitinib treatment, but there was improvement in the symptoms and image findings after changing the treatment to erlotinib.
| Case Report | ▴Top |
The patient was a 54-year-old woman with no history of smoking. A CT scan showed a nodular shadow 2 cm long in the left upper lobe, as well as multiple granular shadows in both lungs. Adenocarcinoma was indicated from a bronchoscopic biopsy of the primary tumor and diagnosed c-T4N0M1 stage IV was diagnosed. Beginning in mid-February, a course of cisplatin and gemcytabine was started, but was discontinued due to progressive disease. Analysis of the EGFR gene revealed an exon 21 L858R point mutation. Therefore, gefitinib at 250 mg was started orally from March, and after one month, resulting in partial response. Administration was continued on an outpatient basis, however, the symptoms of headache and nausea upon rising in the morning started in mid-June. The patient was re-admitted to our department on July 28th on suspicion of brain metastasis.
The Chest CT scan on re-admission showed that the primary tumor in the left upper lobe had been significantly diminished compared to start of treatment and the diffuse granular shadows observed in both lungs during treatment with gefitinib (Fig. 1a, b). The contrast-enhanced cranial MRI on re-admission detected a small high intensity area in the thalamus, a high intensity area around the ventricle and enhanced cerebral surface of the occipital lobe. Therefore brain metastasis and carcinomatous meningitis were suspected (Fig. 2a). Laboratory findings on re-admission revealed an elevated level of CEA (11.6 ng/mL). Cytology of the cerebrospinal fluid revealed a positive result (class V) of adenocarcinoma; a diagnosis of carcinomatous meningitis was made and the PD was therefore determined.
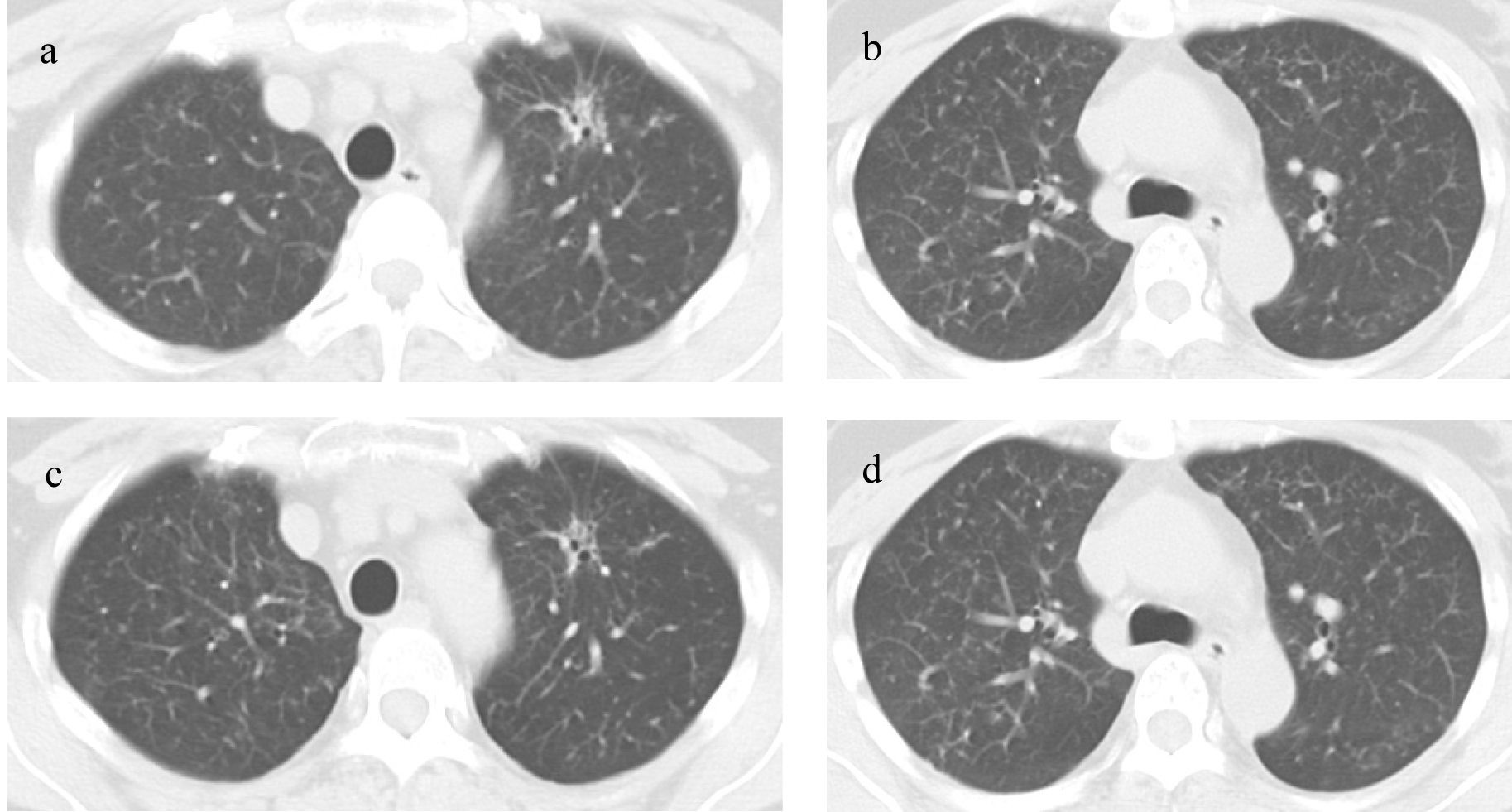 Click for large image | Figure 1. Chest CT scans: (a, b) Chest CT scan on re-admission; (c, d) Chest scan after 8 months of erlotinib treatment. |
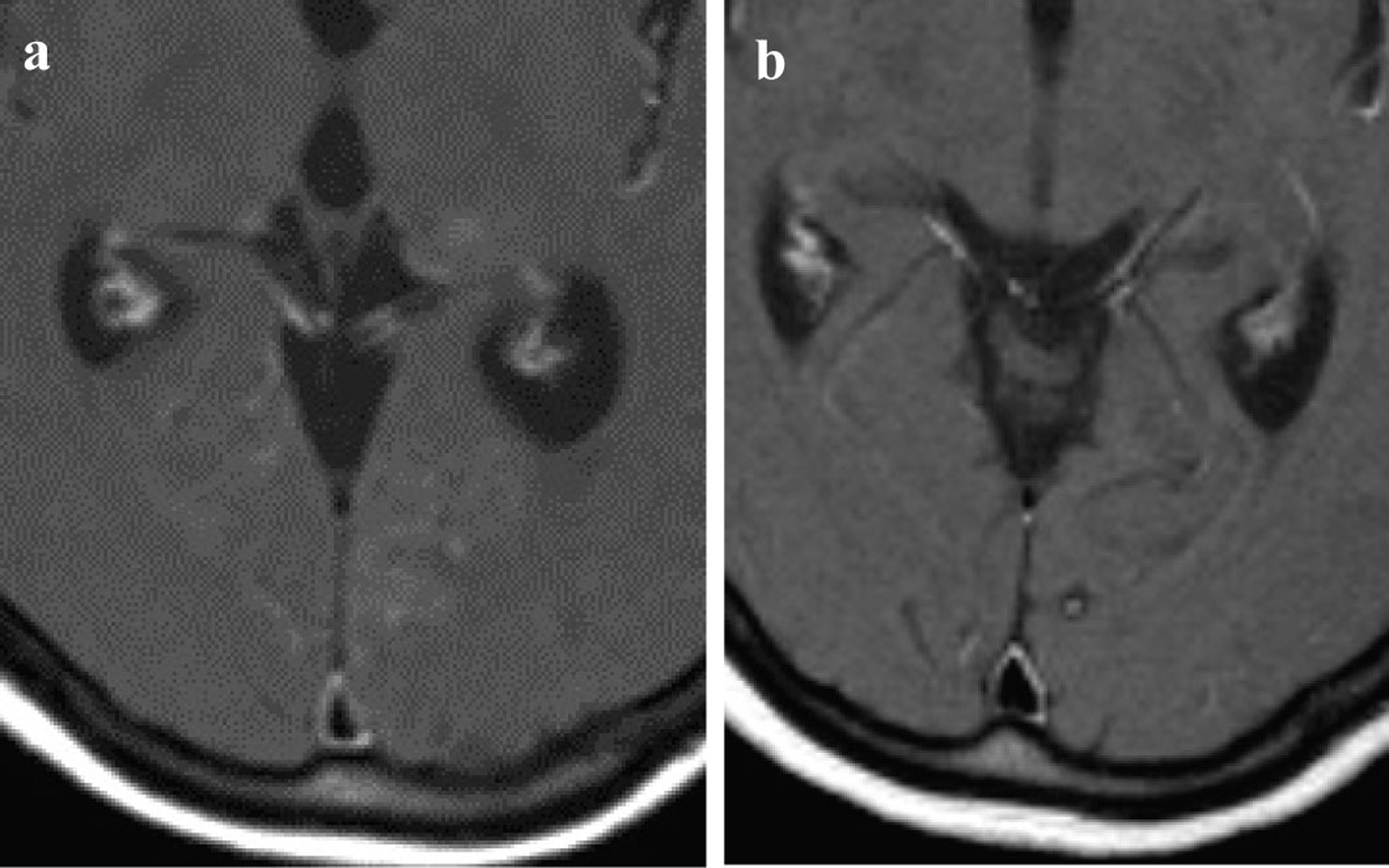 Click for large image | Figure 2. Contrast-enhanced cranial MRI: (a) Contrast-enhanced cranial MRI on re-admission; (b) Contrast-enhanced cranial MRI after 8 months of erlotinib treatment. |
First, whole-brain irradiation (3 Gy/day 10 days) was started for the carcinomatous meningitis, and gefitinib oral administration was continued. After the completion of irradiation of only 9 Gy, disorientation, aphasia, and paresis of the right side of the body suddenly developed. A head CT and contrast-enhanced MRI showed no hemorrhage or infarction; however, cerebral edema was noted. Intracranial hypertension associated with radiation as well as carcinomatous meningitis were possible causes and so administration of glycerol and steroid was started. The aphasia and right-sided paralysis improved immediately and the consciousness gradually returned to a normal level. The brain lesions were not controlled by the combination of whole-brain irradiation and gefitinib, but gefitinib remained effective against metastases other than in the brain, therefore, we concluded that the tumor was still sensitive to the epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), but that the concentration had not attained an effective level in the brain. We changed the treatment from gefitinib to erlotinib (150 mg/day), another EGFR-TKI with a higher plasma concentration. After starting treatment with erlotinib, symptoms such as headache and nausea gradually subsided. CSF analysis after 4 month of erlotinib treatment revealed a negative result for cancer cells. After 8 month of treatment, contrast-enhanced cranial MRI showed that the high intensity regions around the ventricles had regressed and the brain metastatic lesions had also became obscure (Fig. 2b). A chest CT showed that the primary tumor in the left upper lobe was further reduced, and the diffuse granular shadows in both lungs had almost disappeared after (Fig. 1c, d).
The serum CEA level that had tended to show slight increase when carcinomatous meningitis developed during oral administration of gefitinib began to decrease when oral erlotinib treatment was started, and decreased to normalized (Fig. 3). The patient has no symptoms at present other than an occasional headache.
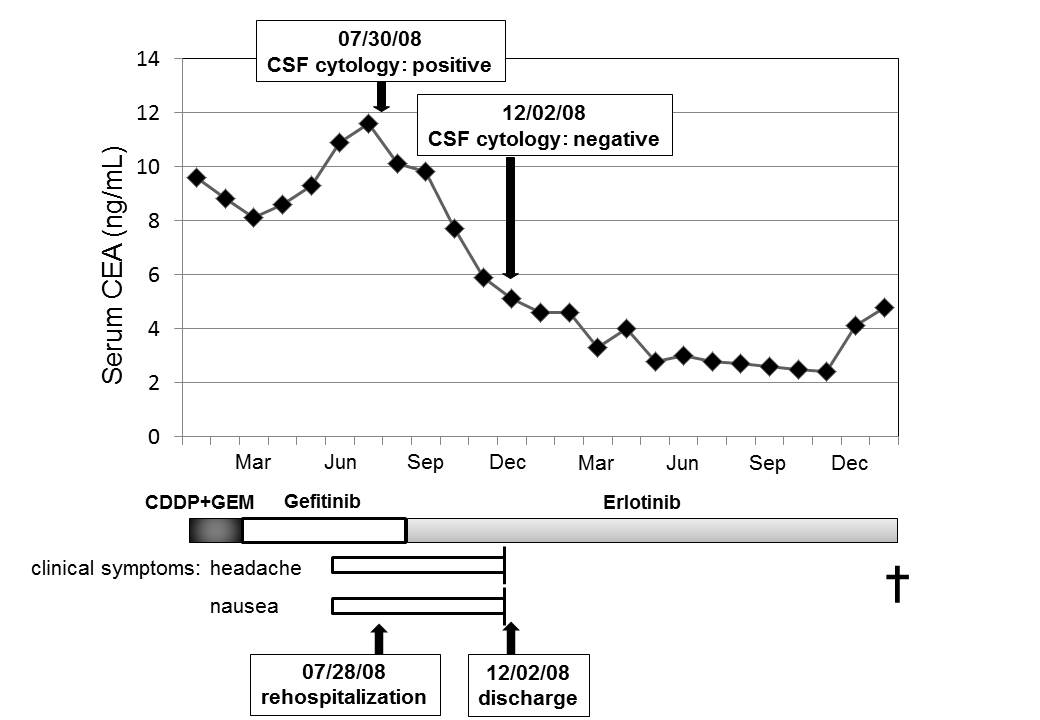 Click for large image | Figure 3. Clinical course of patient suffered from carcinomatous meningitis. |
| Discussion | ▴Top |
Carcinomatous meningitis is caused by tumor cells that have reached the meninges by, for example, hematogeneous dissemination or direct invasion from the tumor. Once the tumor cells reach the subarachnoid space, they spread to the meninges via the cerebrospinal fluid. Then a variety of central neurological symptoms such as headache, loss of consciousness, cognitive symptoms, convulsions, and paralysis, can occur [2]. The prognosis is poor and there are no established treatment protocols at present. In recent years, a few reports have appeared stating that brain metastasis and carcinomatous meningitis responded to gefitinib [3, 4], however, its efficacy is still uncertain.
In our case, even though the lung lesions responded to gefitinib, central nervous system lesions such as brain metastasis and carcinomatous meningitis were exacerbated. However, when the therapy was changed to erlotinib, a response in the central nervous system lesions was noted and the effect on the lung lesions continued. The central nervous system lesions were exacerbated during gefitinib treatment but treatment with another EGFR-TKI, erlotinib, was effective. The result could be due to the difference in the clinical regimes for erlotinib and gefitinib.
H.G.Yi.et al [5] reported a case in which a patient with brain metastasis from lung adenocarcinoma responded well to gefitinib (250 mg) and intraventricularly administerd MTX after whole brain irradiation. Twelve months later, carcinomatous meningitis confirmed by CSF cytology had recovered. The symptoms had improved and no cancer cells were detected. Jackman DM et al [6] found the 50% inhibitory concentration (IC50) of gefitinib in a cancer cell line obtained from the pleural effusion of a patient with EGFR gene exon 19 deletions who developed brain metastasis during oral gefitinib (250 mg) treatment to be 10-50 nmol/L. Then they compared the gefitinib concentration in the CSF with the gefitinib dosage. During the period of a dosage of 500 mg, the CSF concentration was 6.2-18 nmol/L, and lower than the IC50 value. However, as the dosage was increased to 750 mg and 1000 mg, the CSF concentration went up to 32 and 42 nmol/L, respectively. The brain metastasis in this patient was also reduced with the increased gefitinib dosages. This patient died from the progression of the primary lung tumor and liver metastasis. The autopsy detected the expression of the gefitinib resistance gene, T790M [7], in the lung lesion and liver metastasis but it was not in the cancer cells in the CSF. There is another case report in which gefitinib was initially effective but, 14 months later, the brain metastasis was exacerbated, causing symptoms. When the treatment was changed to erlotinib, the symptoms improved and the brain lesions were reduced [8], similar to the coarse of events that occurred with our patient. From the above results, we concluded that the reason for the development of central nervous system lesions during gefitinib therapy appears to arise from a sub-optimal concentration of gefitinib in the CSF, rather than development of resistance. By increasing the gefitinib dosage, a concentration may be obtained that more effectively controls central nervous system lesions. Our patient developed central nervous system lesions even though gefitinib was effective against lung lesions and thus the patient would not have been considered resistant to gefitinib, even though an analysis has not been done.
The clinical dose of gefitinib is set at 250 mg, one third of the maximum tolerated dose. However, the clinical dose of erlotinib is set at 150 mg, which is the maximum tolerated dose [9]. There is no report in the literature comparing the transfer of gefitinib and elotinib into the CSF but dose-dependency has been confirmed for both. Apparently, the clinical dose of erlotinib has a more effective concentration in the CSF.
We encountered a case of lung adenocarcinoma in which central nervous system lesions developed even though the lung lesions were responding to gefitinib, and improvement was seen by changing to erlotinib, a similar EGFR-TKI chemotherapeutic agent. We consider this improvement to be due to the fact that a more effective concentration was achieved with erlotinib, and note the difference in the clinical regimes of gefitinib and erlotinib. Efficacy may be more readily attained by selecting erlotinib rather than gefitinib for patients with lung adenocarcinoma complicated by central nervous system lesions.
| References | ▴Top |
- Chamberlain MC, Kormanik P. Carcinoma meningitis secondary to non-small cell lung cancer: combined modality therapy. Arch Neurol. 1998;55(4):506-512.
pubmed doi - Chamberlain MC. Neoplastic meningitis. J Clin Oncol. 2005;23(15):3605-3613.
pubmed doi - Kim MK, Lee KH, Lee JK, Choi JH, Hyun MS. Gefitinib is also active for carcinomatous meningitis in NSCLC. Lung Cancer. 2005;50(2):265-269.
pubmed doi - Fukuhara T, Saijo Y, Sakakibara T, Inoue A, Morikawa N, Kanamori M, Nakashima I, et al. Successful treatment of carcinomatous meningitis with gefitinib in a patient with lung adenocarcinoma harboring a mutated EGF receptor gene. Tohoku J Exp Med. 2008;214(4):359-363.
pubmed doi - Yi HG, Kim HJ, Kim YJ, Han SW, Oh DY, Lee SH, Kim DW, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009;65(1):80-84.
pubmed doi - Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, Bailey C, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24(27):4517-4520.
pubmed doi - Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786-792.
pubmed doi - Walther JC, Khorshid M, Gaya A, Plowman PN. Cross-over response to erlotinib of brain metastatic disease from bronchial adenocarcinoma after gefitinib failure, and an unusual rash. Clin Oncol (R Coll Radiol). 2006;18(8):637-639.
pubmed doi - Comis RL. The current situation: erlotinib (Tarceva) and gefitinib (Iressa) in non-small cell lung cancer. Oncologist. 2005;10(7):467-470.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


