| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 5, Number 4, April 2014, pages 187-193
Lead Intoxication Treated With D-Penicillamine
Yuka Iijimaa, Nobuhiro Akuzawaa, c, Takashi Hatoria, Kunihiko Imaia, Yonosuke Kitaharaa, Masahiko Kurabayashib
aDepartment of Internal Medicine, Social Insurance Gunma Chuo General Hospital, 1-7-13 Koun-cho, Maebashi, Gunma 371-0025, Japan
bDepartment of Medicine and Biological Science, Gunma University Graduate School of Medicine, 3-39-22 Showa-machi, Maebashi, Gunma 371-8511, Japan
cCorresponding author: Nobuhiro Akuzawa, Social Insurance Gunma Chuo General Hospital, 1-7-13 Koun-cho, Maebashi, Gunma 371-0025, Japan
Manuscript accepted for publication February 11, 2014
Short title: Lead Intoxication
doi: https://doi.org/10.14740/jmc1698w
| Abstract | ▴Top |
A 36-year-old man was admitted to our hospital with abdominal pain, nausea, weight loss and malaise. He had worked as a painter for 2 months. Laboratory testing showed anemia, liver dysfunction and elevated blood lead concentration. After administration of oral D-penicillamine, his urinary lead excretion increased significantly. His abdominal pain resolved after 5 days. His clinical course was otherwise uneventful. His anemia and liver dysfunction resolved after 2 months. His blood lead concentration remained normal for a year after discharge without recurrence of plumbism. Physicians should be aware of the clinical features of plumbism because early diagnosis is difficult.
Keywords: Abdominal pain; Anemia; D-penicillamine; Lead intoxication
| Introduction | ▴Top |
High lead concentrations can affect a wide range of biological functions leading to gastrointestinal dysfunction, anemia, neurological dysfunction, hypertension and kidney damage [1]. Inhalation or ingestion of lead particles results in lead intoxication. Inhalation of lead fumes or dust may cause lead intoxication in workers doing painting, paint removal or construction [1-3]. The symptoms of acute intoxication may be subtle and non-specific, and patients may present with musculoskeletal pain, gastrointestinal complaints, headache, fatigue and hematological abnormalities [3]. Chronic lead intoxication may cause irreversible end-organ damage in the nervous system, endocrine glands, kidneys, joints and bones [3].
A diagnosis of lead intoxication is often overlooked because the blood lead concentration does not reflect body lead stores [2]. Increased urinary lead excretion after calcium-ethylenediaminetetraacetic acid (Ca-EDTA) administration indicates high body lead stores [2]. Chelating agents such as Ca-EDTA, D-penicillamine, dimercaprol (BAL) or succimer (meso-2,3-dimercaptosuccinic acid, DMSA) can be used to treat lead intoxication [3, 4].
Here, we present a male patient who developed lead intoxication after working as a painter for 2 months. His main complaints were loss of appetite, constipation and nausea. The main cause of his lead intoxication was probably inhalation of lead particles while stripping anti-rust compounds including Pb3O4 from a bridge. His symptoms rapidly improved after chelation therapy with D-penicillamine. This report focuses on the important symptoms for diagnosis of lead intoxication and reviews previously reported cases, including discussion of the difficulty of early diagnosis.
| Case Report | ▴Top |
A 36-year-old man was admitted to our hospital because of increasing fatigue, constipation and nausea. He had been a clerical worker until 3 months before admission, but the bankruptcy of his company had forced him to change jobs. He had therefore started work as a painter 2 months before admission. His main duties were paint stripping and re-painting of a bridge. He wore a helmet, goggles, dust respirator, protective clothing and leather gloves. He started to develop intermittent abdominal pain after 3 weeks and was prescribed scopolamine butylbromide tablets at another hospital, but his pain did not resolve. His body weight decreased from 55 kg to 48 kg over 8 weeks and he complained of persistent malaise, nausea and loss of appetite, and was admitted to our hospital for a detailed assessment. He had no remarkable past history or family history. He was a nonsmoker and did not drink alcohol. He was taking only mosapride and lansoprazole, which had been prescribed at another hospital.
Physical examination on admission revealed that his height was 165 cm, weight was 48 kg, temperature was 37.3 °C and heart rate was 78 beats/min. He was hypertensive, with a blood pressure of 155/84 mmHg. He had moderate conjunctival pallor, gray pigmentation of the gingiva and hyperactive bowel sounds, but no abdominal tenderness. Neurological examination showed no abnormalities. Electrocardiography and chest X-ray were normal, and abdominal X-ray showed increased intestinal gas and constipation (Fig. 1A). Routine laboratory testing on admission showed normocytic normochromic anemia, mild liver dysfunction, hyponatremia and a high blood urea nitrogen level (Table 1). His serum iron and haptoglobin levels were normal. His unsaturated iron binding capacity was low despite a high serum ferritin level, suggesting abnormal iron utilization. He had normal serum levels of vitamin B12 (486 pg/mL, normal range: 180 - 914 pg/mL) and folic acid (5.4 ng/mL, normal range: 2.4 - 9.7 ng/mL). His blood smear showed basophilic stippling (Fig. 1B) and a high reticulocyte count (4.1%). His urinary hippuric acid, methylhippuric acid and 2,5-hexanedione levels were not elevated. We measured his blood lead, chromium and cadmium concentrations because his history suggested possible inhalation or ingestion of stripped paint dust including these heavy metals. His blood chromium concentration was < 2 µg/dL and cadmium concentration was < 1 µg/dL, but his blood lead concentration was high (111 µg/dL, normal range: < 20 µg/dL).
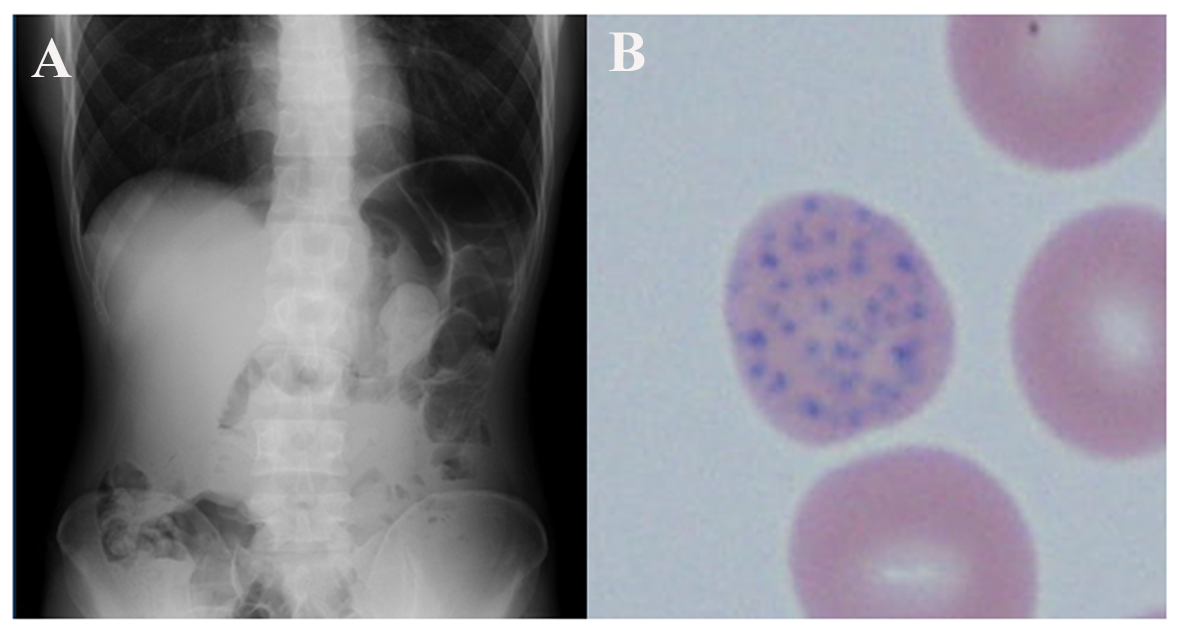 Click for large image | Figure 1. (A) Abdominal X-ray on admission showed retention of feces suggesting constipation. There were no other abnormalities despite severe abdominal pain. (B) Blood smear on admission showed basophilic stippling in erythrocytes, leading to a suspicion of lead intoxication (original magnification × 400). |
 Click to view | Table 1. Laboratory Findings on Admission |
The diagnosis of lead intoxication was confirmed with a positive lead chelation test. We initially intended to administer Ca-EDTA as a first-line chelating agent, but it would have taken about a week to obtain this. Instead, we administered 1 g/day of D-penicillamine orally for a week from day 2. His urinary lead excretion measured from pooled urine samples rose from 0.9 mg/day on day 2 to 5.1 mg/day on day 3, and decreased thereafter (Fig. 2). The δ-aminolevulinic acid (δ-ALA) concentration in spot urine samples peaked on day 2 and then gradually decreased. His abdominal pain resolved by day 5, and he was able to tolerate a normal diet by day 10. His constipation improved over the same period. From day 7, his urinary lead excretion measured from pooled urine samples stayed less than 0.5 mg/day, and his δ-ALA concentration in spot urine samples stayed below 15 mg/L. We therefore stopped chelation therapy on day 8. Although his anemia and associated symptoms such as shortness of breath on effort persisted, he was discharged from hospital on day 14, because his hemoglobin concentration was gradually improving. He was moved to the sales department of his company, where there was no possibility of further exposure to lead. His blood lead concentration was 26.3 µg/dL at 1 month after discharge and 15.9 µg/dL at 2 months. His δ-ALA concentration in spot urine samples also normalized to 2.1 mg/L after 2 months. His hemoglobin concentration increased to 10.0 g/dL after 1 month, and returned to normal (14.5 g/dL) after 2 months. His serum ferritin level decreased to 212.3 ng/mL after 1 month and normalized to 69.6 ng/mL after 2 months, and his liver dysfunction resolved after 2 months. The basophilic stippling in his blood smear also resolved after 2 months. He was well at his 1-year follow-up assessment, with no recurrence of plumbism.
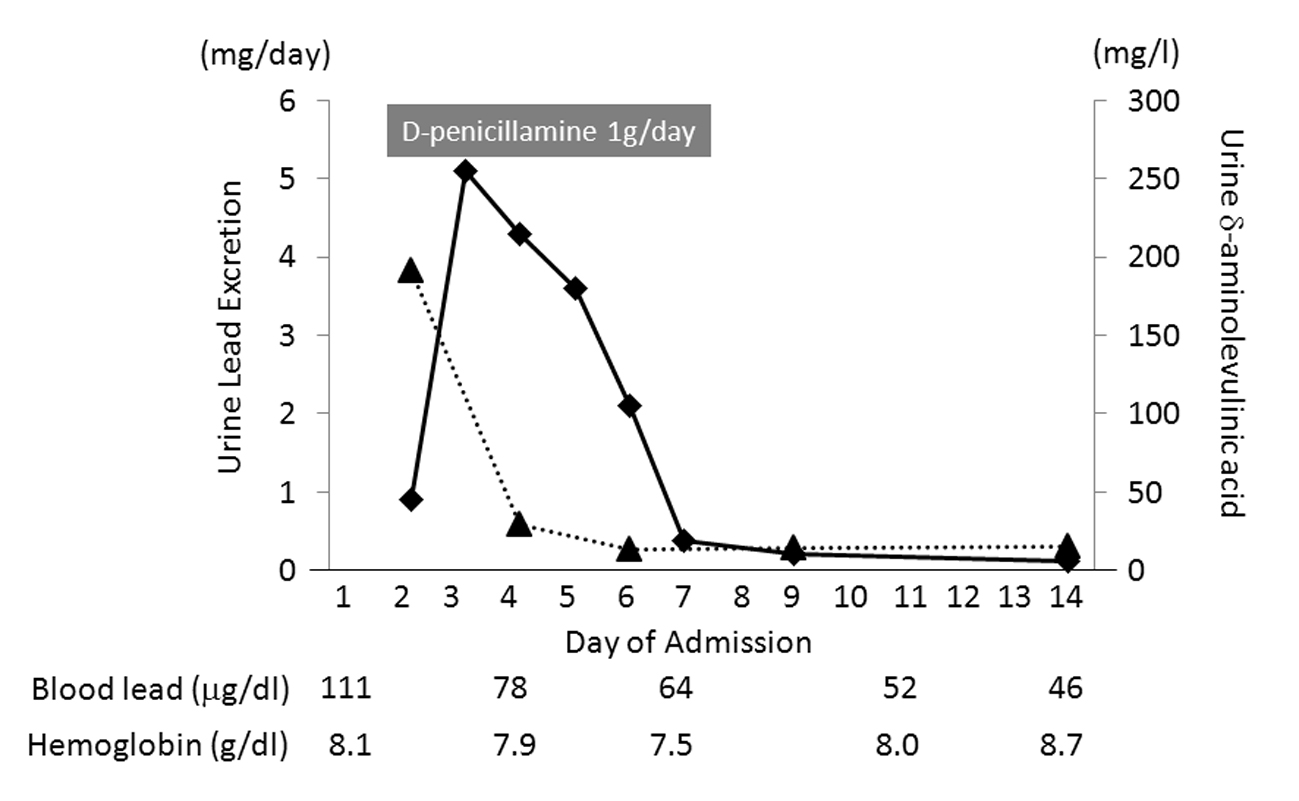 Click for large image | Figure 2. Daily urinary lead excretion (rectangles) and δ-aminolevulinic acid (δ-ALA) concentration in spot urine samples (triangles). The δ-ALA concentration peaked on day 2. Lead excretion increased significantly after administration of D-penicillamine, peaking on day 3. Both parameters gradually decreased after the peak. |
| Discussion | ▴Top |
The most common clinical picture of plumbism is of chronic lead intoxication, often due to occupational exposure over a prolonged period [5]. The blood lead concentration reflects lead distribution in the red blood cells and plasma and may be useful for the diagnosis of plumbism, but lead is also distributed in the soft tissues and bones [6]. The active pool is distributed in the blood and soft tissues and the storage pool is in the bones [6]. The half-life of lead is 35 days in the blood, 40 days in the soft tissues and 20 - 30 years in the bones [6]. The blood lead concentration may therefore not be an accurate reflection of previous lead absorption if the exposure is not continuous and ongoing. Moreover, many patients who presented with severe lead poisoning had lower blood lead concentrations (< 40 µg/dL) [7], suggesting a need for other indicators for early-stage diagnosis of plumbism. Lead is an electropositive metal with a high affinity for sulfhydryl groups, and inhibits 5-aminolevulinic acid dehydratase and ferrochelatase [8]. A high urinary δ-ALA concentration or blood zinc protoporphyrin level may therefore be useful for diagnosing lead intoxication, especially in patients with an acceptable blood lead concentration despite symptoms of lead intoxication. Furthermore, re-elevation of the urinary δ-ALA level due to tissue redistribution of lead can indicate the need for additional administration of chelating agents [9]. Measurement of the total urinary lead excretion the day after Ca-EDTA infusion (30 mg/kg, up to 2 g) is the most direct method of estimating the total body lead burden, and this test is positive if a 24-h urine collection after chelation contains more than 2.89 µmol (0.6 mg) [3]. In the present case, we administered D-penicillamine instead of Ca-EDTA and the 24-h urinary lead excretion peaked at 5.1 mg/day. Until recently, Ca-EDTA and BAL were the main drugs used for the treatment of lead intoxication in adults [10]. Both these drugs are not effective when taken orally, and should be administered intravenously [10]. Ca-EDTA is also reported to remove substantial amounts of zinc, an essential metal, from the body [11]. More recently, succimer (DMSA), a water-soluble analogue of BAL that can be administered orally, has been used for the treatment of heavy metal poisoning including lead, arsenic and mercury [12]. D-penicillamine is another chelating agent that can be taken orally, but is not as effective as EDTA or BAL, and is associated with serious adverse events including nephrotic syndrome, severe bleeding and leukopenia [10]. The results of a recent study suggest that the optimal regimen for D-penicillamine administration in lead poisoning is 25 - 35 mg/kg/day in divided doses for 3 weeks, with a gap of 7 days between courses [13]. Two or three courses of D-penicillamine are often needed to reduce the blood lead concentration to an acceptable level [13, 14]. Fortunately, our patient did not develop any complications related to D-penicillamine therapy, and there was no re-elevation of his urinary δ-ALA concentration or blood lead concentration after 7 days of chelation therapy. This suggests that a single course of oral D-penicillamine can provide sufficient chelation of lead in patients with relatively low body lead levels.
A previous review of adult cases of inorganic lead intoxication reported 31 cases due to occupational exposure [15]. Of these, 29% had no complaints, and the most common symptom in the remainder was abdominal pain, followed by fatigue and arthralgia. All six patients who presented with acute-onset symptoms had abdominal pain. Moreover, loss of appetite and weakness are more common in patients with plumbism than in healthy adults [14]. We searched the PubMed database from 2000 to 2013, and found 10 reported patients with acute-onset symptoms of lead intoxication (Table 2) [5, 8, 16-20]. Six of these 10 patients had occupational lead exposure, and nine underwent chelation therapy. The period of lead exposure was reported in only three cases. Although most of the patients had symptoms such as abdominal pain, malaise, headache, nausea and constipation, they did not seek treatment from a hospital or their local doctor while they could still tolerate the symptoms, leading to delays in diagnosis and treatment [17]. In five patients (cases 5, 6, 8, 9 and 10), abdominal pain led to consultation with a doctor even though preceding nonspecific complaints such as fatigue, malaise and headache had been tolerated [5, 17, 19-21]. None of the reported cases had severe encephalopathy. Routine hematology and biochemistry testing may show anemia, which is typically hypochromic and microcytic and generally appears when the blood lead concentration exceeds 50 µg/dL [8]. Anemia is therefore not observed in all patients with lead intoxication, and bone marrow biopsy does not show specific findings [18]. It should be noted that the hemoglobin concentration was above 10 g/dL in three cases (cases 3, 5 and 6) despite high blood lead concentrations [8, 15, 17]. Basophilic stippling, caused by aggregation of ribonucleic acid within erythrocytes due to inhibition of pyrimidine 5’-nucleotidase [3, 8], was observed in seven of the cases. Although basophilic stippling was also found in 27% of consecutive internal medicine patients without lead exposure and in patients with thalassemia [18, 22], it is worth remembering that this finding may indicate lead intoxication [19]. An elevated urinary δ-ALA or porphyrin level in the absence of urinary porphobilinogen may enable definitive diagnosis of lead intoxication, and diagnostic testing for porphyria is therefore recommended [5, 18]. A high blood zinc protoporphyrin level is also useful for diagnosis [13, 19]. Increased serum alanine aminotransferase or creatinine levels may be observed [5, 20, 21], but these findings are also nonspecific. In some patients, especially those with no obvious history of occupational lead exposure, diagnosis may be delayed from the time of first presentation with symptoms and it may take several months to start appropriate treatment for lead intoxication [16]. In case 1, the occasional use of a mug with a ceramic inner surface that contained lead caused intoxication [16]. In case 8, ingestion of medication purchased online for erectile dysfunction led to acute lead intoxication [5]. A recent study also reported cases of lead intoxication due to regular use of lead-contaminated opium (case 9) or alternative medications (case 10) [19, 20]. Physicians should therefore be aware that lead intoxication can be associated with food preparation or storage habits, medications and opium addiction, as well as occupational lead exposure.
 Click to view | Table 2. Reported Adult Patients with Acute-Onset Symptoms of Lead Intoxication |
In conclusion, we reported a patient who presented with lead intoxication after 2 months of occupational lead inhalation despite appropriate use of a respirator. Chelation therapy with oral D-penicillamine was effective and his clinical course was good. Abdominal pain is the most frequent symptom in patients with acute-onset symptoms of lead intoxication, and concomitant symptoms are nonspecific for making a diagnosis. Thus, it may be difficult for physicians to make a rapid diagnosis of lead intoxication. Physicians should be aware of the possibility of lead intoxication and the potential causes of plumbism when investigating patients with frequent abdominal pain with no obvious cause.
Acknowledgments
The authors would like to thank Mr. Yousuke Kitadume for his assistance in preparing blood smear samples and Miss Hisae Kuribara for her secretarial assistance under the financial support provided by Social Insurance Gunma Chuo General Hospital.
Grant Support
None.
Financial Disclosure
All the authors report no conflicts of interest.
| References | ▴Top |
- International Program on Chemical Safety (IPCS). Environmental Health Criteria 379. Geneva: WHO, 2013.
- Pollock CA, Ibels LS. Lead intoxication in paint removal workers on the Sydney Harbour Bridge. Med J Aust. 1986;145(11-12):635-639.
pubmed - Rae CE, Bell CN
Jr , Elliott CE, Shannon M. Ten cases of acute lead intoxication among bridge workers in Louisiana. DICP. 1991;25(9):932-937.
pubmed - Rempel D. The lead-exposed worker. JAMA. 1989;262(4):532-534.
doi pubmed - Barber T, Jacyna M. Acute lead intoxication from medications purchased online presenting with recurrent abdominal pain and encephalopathy. J R Soc Med. 2011;104(3):120-123.
doi pubmed - Grimsley EW, Adams-Mount L. Occupational lead intoxication: report of four cases. South Med J. 1994;87(7):689-691.
doi pubmed - Beritic T. Lead concentration found in human blood in association with lead colic. Arch Environ Health. 1971;23(4):289-291.
doi pubmed - Gordon JN, Taylor A, Bennett PN. Lead poisoning: case studies. Br J Clin Pharmacol. 2002;53(5):451-458.
doi pubmed - Molina-Ballesteros G, Zuniga-Charles MA, Sanchez-Anzaldo FJ, Gonzalez-Ramirez JD. Urinary delta-aminolevulinic acid as a biological indicator throughout penicillamine therapy in lead intoxication. Arch Environ Health. 1978;33(6):308-313.
doi pubmed - Llobet JM, Domingo JL, Paternain JL, Corbella J. Treatment of acute lead intoxication. A quantitative comparison of a number of chelating agents. Arch Environ Contam Toxicol. 1990;19(2):185-189.
doi pubmed - Chisolm JJ
Jr . Treatment of acute lead intoxication—choice of chelating agents and supportive therapeutic measures. Clin Toxicol. 1970;3(4):527-540.
doi pubmed - Aposhian HV. DMSA and DMPS—water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983;23:193-215.
doi pubmed - Dsouza HS, Dsouza SA, Menezes G, Thuppil V. Evaluation and treatment of wrist drop in a patient due to lead poisoning: case report. Ind Health. 2009;47(6):677-680.
doi pubmed - D'Souza H S, Dsouza SA, Menezes G, Venkatesh T. Diagnosis, evaluation, and treatment of lead poisoning in general population. Indian J Clin Biochem. 2011;26(2):197-201.
doi pubmed - Cullen MR, Robins JM, Eskenazi B. Adult inorganic lead intoxication: presentation of 31 new cases and a review of recent advances in the literature. Medicine (Baltimore). 1983;62(4):221-247.
doi - Ziegler S, Wolf C, Salzer-Muhar U, Schaffer A, Konnaris C, Rudiger H, Osterode W. Acute lead intoxication from a mug with a ceramic inner surface. Am J Med. 2002;112(8):677-678.
doi - Ogawa M, Nakajima Y, Kubota R, Endo Y. Two cases of acute lead poisoning due to occupational exposure to lead. Clin Toxicol (phila). 2008;46(4):332-335.
doi pubmed - Dounias G, Rachiotis G, Hadjichristodoulou C. Acute lead intoxication in a female battery worker: Diagnosis and management. J Occup Med Toxicol. 2010;5:19.
doi pubmed - Verheij J, Voortman J, van Nieuwkerk CM, Jarbandhan SV, Mulder CJ, Bloemena E. Hepatic morphopathologic findings of lead poisoning in a drug addict: a case report. J Gastrointestin Liver Dis. 2009;18(2):225-227.
pubmed - Shamshirsaz AA, Yankowitz J, Rijhsinghani A, Greiner A, Holstein SA, Niebyl JR. Severe lead poisoning caused by use of health supplements presenting as acute abdominal pain during pregnancy. Obstet Gynecol. 2009;114(2 Pt 2):448-450.
doi pubmed - Carton JA, Maradona JA, Arribas JM. Acute-subacute lead poisoning. Clinical findings and comparative study of diagnostic tests. Arch Intern Med. 1987;147(4):697-703.
doi pubmed - Cheson BD, Rom WN, Webber RC. Basophilic stippling of red blood cells: a nonspecific finding of multiple etiology. Am J Ind Med. 1984;5(4):327-334.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


