| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 5, Number 2, February 2014, pages 83-88
Use of a Daily Lung Compliance Evaluation to Track Progress on ECMO: Successful Use in a Patient With Cystic Fibrosis
Samer Abu-Sultaneha, c, Shekhar S. Raja, Brian D. Benneywortha, A. Ioana Cristeab, Mara E. Nitua, Mark R. Rigbya
aDepartment of Pediatrics, Section of Pediatric Critical Care Medicine, Indiana University School of Medicine and Riley Hospital for Children at Indiana University Health, Indianapolis, IN, USA
bDepartment of Pediatrics, Section of Pediatric Pulmonary, Indiana University School of Medicine and Riley Hospital for Children at Indiana University Health, Indianapolis, IN, USA
cCorresponding author: Samer Abu-Sultaneh, Pediatric Critical Care Indiana University School of Medicine and Riley Hospital for Children at Indiana University Health, 705 Riley Hospital Drive, ROC 4905, Indianapolis, Indiana, USA
Manuscript accepted for publication October 2, 2013
Short title: Cystic Fibrosis
doi: https://doi.org/10.14740/jmc1511w
| Abstract | ▴Top |
Survival rates of extracorporeal membrane oxygenation (ECMO) for pediatric respiratory failure have been improving and are now about 70%. With this, traditional exclusionary criteria for ECMO may be challenged. We hypothesize that an objective evaluation of pulmonary recovery whilst on ECMO may assist in the care of high risk patients, such as those with cystic fibrosis (CF), both to strategize appropriate decannulation and avoid futility. A 19-year-old female with CF developed septic shock and MRSA-associated acute respiratory distress syndrome. After 4 days, her respiratory status deteriorated and was transitioned to veno-venous ECMO. Due to uncertainty of pulmonary recovery and survival, we instituted a “daily lung compliance trial” (DLCT) to objectively assess pulmonary compliance and function. This included increasing ventilatory support from “rest settings” to moderate non-toxic setting and assessing pulmonary pressures and compliances after 30 min. This provided objective data of lung healing. Due in part to this data, the patient was decannulated from ECMO after 11 days and successfully extubated 2 days later. ECMO can be used for CF patients with acute respiratory failure as a bridge to recovery. Using a DLCT can help guide decision making for respiratory ECMO patients with significant co-morbidities.
Keywords: Extracorporeal membrane oxygenation; Extracorporeal life support; Cystic fibrosis; Lung compliance; Acute respiratory distress syndrome; Extracorporeal life support organization
| Introduction | ▴Top |
The combination of the advances in technology and experience using extracorporeal membrane oxygenation (ECMO) made over the past three decades are now challenging the traditional concepts of patient eligibility for this life-saving technology [1-3]. Such advances include improvements in cannulation techniques, pumps and oxygenator technology, and anticoagulation management. Given these technical advances, the success of ECMO has improved substantially, increasing the survival of pediatric respiratory ECMO from 57% to 72% over the past two decades [4, 5]. The traditional absolute or relative contraindications for ECMO (including congenital or acquired immune deficiencies and underlying respiratory disease) are now challenged. Because of the irreversibility of the underlying condition, traditionally, ECMO may have been denied to patients with cystic fibrosis (CF) with severe, unrelenting, acute respiratory failure and multisystem organ disease.
Improvement of the care of CF patients has led to a growing population of patients with minimal degrees of pulmonary and multi-organ dysfunction. Coupled with the aforementioned advances in ECMO, it is presently unclear if and when patients with CF should be offered ECMO; and, if so, how to assess lung recovery during support.
We report a case of teenage girl with CF who developed septic shock, ARDS and MODS successfully supported to survival with VV-ECMO. We used a simple, yet novel, approach to assess lung recovery while on ECMO which helped determine the trajectory of recovery and appropriateness for decannulation.
| Case Report | ▴Top |
A 19-year-old girl with CF and CF-related pancreatic deficiency and CF related diabetes was admitted to our ICU with vomiting, lethargy and increase work of breathing. Soon after admission she was intubated, fluid resuscitated and placed on vasoactive support (dopamine, epinephrine and norepinephrine) for presumed septic shock. She became oliguric and her blood urea nitrogen and creatinine became elevated (eventually 43 mg/dL and 3.9 mg/dL respectively). She was placed on continuous renal replacement therapy (CRRT) on ICU day 3. Stool PCR was positive for Clostridium difficile and endotracheal tube aspirate culture was positive for methicillin-resistant Staphylococcus aureus (MRSA). Due to worsening respiratory status on ICU day 4 she was placed on high frequency oscillatory ventilation (HFOV) with significant support (FiO2 1.0, mean airway pressure 34, amplitude 98, and frequency 4 Hz). The patient continued to suffer from shock, severe hypoxemia (P/F ratio of 49 and oxygenation index of 63), worsening bilateral lung infiltrates, and a combined respiratory and metabolic acidosis (arterial pH 7.22, PaCO2 61 with a base deficit of 3).
Due to concerns of additional ongoing ventilator associated lung injury, our intensivist and pulmonary care teams discussed the appropriateness of ECMO. In further review, prior to this acute event, our patient had mild respiratory disease (including FEV1 of 64%, no home oxygen needs) with good medical compliance, only two CF exacerbations the past year, and no neurological issues. Because this seemed like a rare and unconventional indication for ECMO, we conducted a literature review and found only one published case of CF patient that survived ARDS using ECMO without the need for lung transplant [6]. Given her irreversible underling respiratory diagnosis, she might not be considered an optimal ECMO candidate. Yet after family centered multidisciplinary consultation, it was agreed that the potential benefits outweighed the risks. On ICU Day 4 VV ECMO was initiated without complications. She was transitioned to nominal ventilator “rest settings” (SIMV-PC, PIP = 26, PEEP = 12, Rate = 8, iT = 1.2, FiO2 = 0.5). Over the first few days, the patient continued to require significant ECMO support (namely pump flows of 65 - 90 mL/kg/min, sweep gas at 5 - 8 L/min and FiO2 of 1.0 with post membrane PaO2 around 400).
To aid in pulmonary clearance, serial bronchoscopies were initiated (ECMO day 2, 3 and 5), and nebulized albuterol, acetylcysteine, dornase alfa, hypertonic saline and regular manual hyperinflation were instituted. Intravenous methylprednisolone was started on ECMO day 6. Antibiotic therapy (intravenous vancomycin, metronidazole, piperacillin-tazobactam, tobramycin and enteral vancomycin) was continued.
The approach to weaning ECMO and assessing for decannulation readiness differs between the members of our physician group. As a result, this potentially long ECMO run could have raised several challenges in maintaining the continuity of care. Developing a tool that could objectively assess pulmonary function would be of major importance to care. Thus we developed a serial, standardized approach to regularly document respiratory function and, hopefully, recovery while on ECMO. The “daily lung compliance trial” (DLCT) consists of a temporary increase in the MV settings from the low “rest” to moderate (still non-toxic) settings; SIMV-PRVC with tidal volume of 6 mL/kg, PEEP 12, PS 10, rate 12, iT 1.2 second, FiO2 0.5 for total of 30 minutes. At the end of the DLCT, peak inspiratory pressure (PIP), plateau pressure (Pplat), mean airway pressure (Paw), arterial blood gas, oxygen saturation (SpO2), and end tidal CO2 (EtCO2) were obtained and oxygenation index (OI), static compliance (Cstat) and dynamic compliance (Cdyn) were calculated.
DLCT was initiated on ECMO day 6. They were well tolerated with arterial pH 7.23 - 7.46, PaO2 53 - 69 mmHg, PaCO2 34 - 73 mmHg, SpO2 85-94%, EtCO2 27 - 52, and OI 11.6 - 16.9. The DLCT proved to be a valuable tool to trend pulmonary improvement. The peak and plateau pressures and lung compliance increases of 3 - 4 fold (Fig. 1) were recorded. This data contributed to our decision to decannulate on ECMO day 11. She was extubated 3 days later (ICU day 15) and was discharged home with no supplemental oxygen after 39 days of hospitalization with FEV1 of 46%. Figure 2 shows the radiological course of patient’s lung during hospitalization.
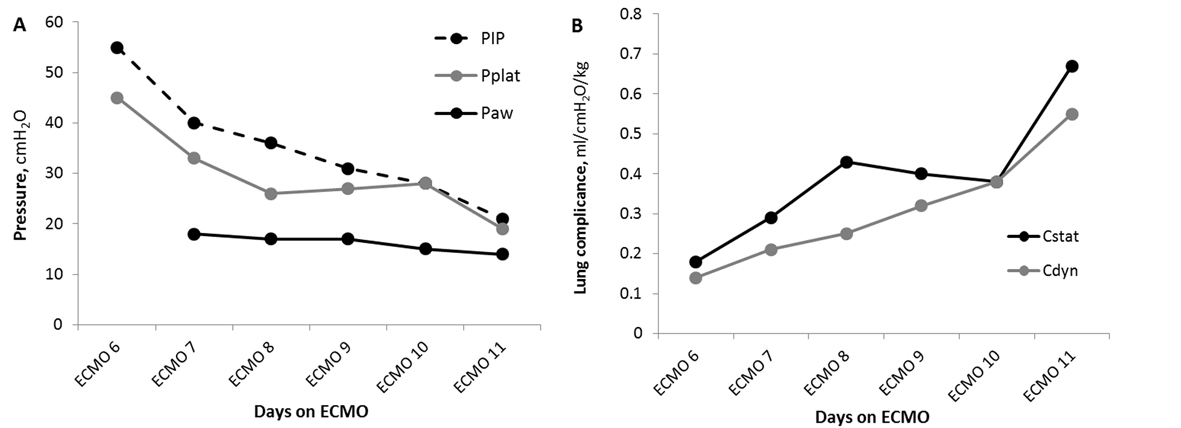 Click for large image | Figure 1. Daily lung compliance trial (DLCT) gives insight to pulmonary improvement on ECMO. Pulmonary pressures (A) and lung compliance (B) during DLCT. Reading (circles) were obtained 30 minutes after initiating DLCT. Cdyn: dynamic compliance; Cstat: static compliance; Paw: mean airway pressure; PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; Pplat: plateau pressure. |
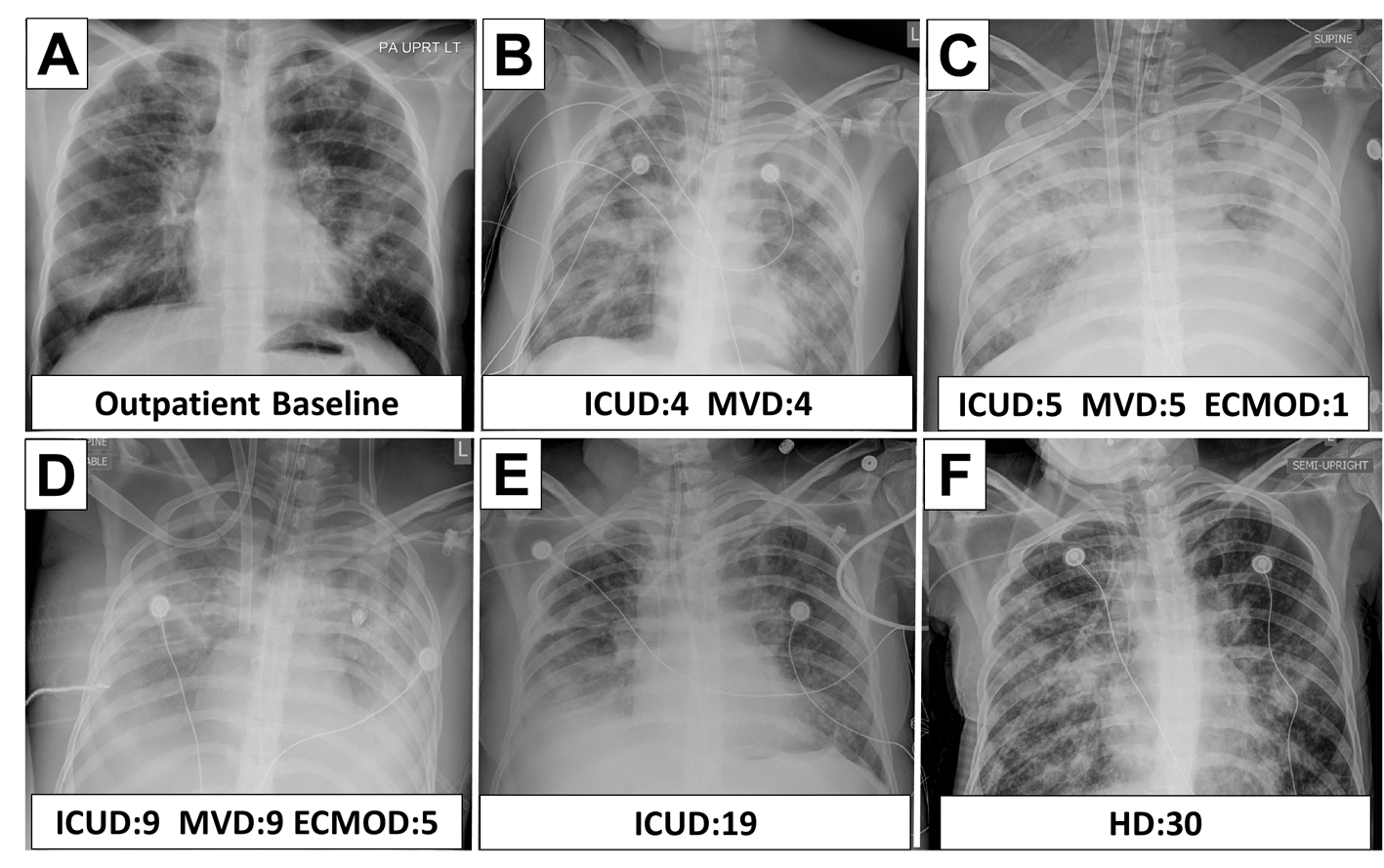 Click for large image | Figure 2. Radiographic course of cystic fibrosis patient while on ECMO. ICUD: ICU day; MVD: mechanical ventilation day; ECMOD: ECMO day; HD: hospital day. |
| Discussion | ▴Top |
ECMO is a rescue therapy for select patients with severe respiratory failure [1-5] that facilitates rest for the lungs and recovery by using non-toxic ventilatory settings while providing adequate gas exchange [7]. With improved technology, experience and success, ECMO is increasingly offered to patients with significant comorbidities, some of which might have been considered contraindications in the past [4, 5]. For cystic fibrosis, ECMO has been successfully used as a bridge for lung transplantation in end stage lung disease [8, 14]. Scant data are available regarding the use of ECMO for CF patients without the plan to use this as a bridge to lung transplantation [6]. Patients with pre-existing chronic lung disease are at a greater risk for acquiring additional ventilator associated lung injury. As a result, CF patients (without a transplant plan) may be considered unsuitable candidates for ECMO.
Our query of the ELSO registry found only 91 CF patients supported with ECMO 1998 to mid-2012, with about half of the runs non-transplant related (Table 1). Survival in transplant-related CF ECMO appears to have improved (namely 45 to 70% before versus after 2007) yet the survival rate has not changed for non-transplant related case (about 40%) despite an increase in ECMO runs. In assessing the limited data on non-transplant related CF ECMO, it appears that young adulthood (18 - 35 years), sepsis and time to initiate ECMO after starting mechanical ventilation (namely < 12 hours or > 2 days) may be risk factors for increased mortality on ECMO (Table 2). It is unclear how inotropic, CVVH, HFOV use or the mode of ECMO (VA versus VV) influences outcome. The length of our patient’s ECMO run was consistent with the median duration of ECMO runs for survivors of 10.3 days (IQR 5.6 - 17.9 days) vs. 8 days (IQR 2.9 - 18.1 days) for non-survivals. Due to the underlying disease of our patient, we expected longer run times, yet this data may suggest that if a CF patient will improve on ECMO they will do so within about 1 - 2 weeks. If not, prognosis may be poor. Needless to say, we believe going onto ECMO, our patient met many “high-risk” categories and thus we were warranted in our significant concern about her ability to recover.
 Click to view | Table 1. Survival Over Time for CF Patients in ELSO Database by Transplant status* |
 Click to view | Table 2. Survival of Non-Transplant CF Patients Requiring ECMO Based on Clinical Characteristics |
To obtain objective data, decrease variability between our physicians, and better understand the trajectory of our patient’s course, we developed a regular “daily lung compliance trial” to standardize assessment of lung recovery. We intermittently assessed compliance (namely Cstat and Cdyn) on reasonable, and justifiably non-toxic, ventilatory settings, rather than just observing tidal volume over time on very low, and arguably insufficient, “rest settings”. DLCT also give a quantitative assessment of gas exchange provided by recovering lungs by obtains blood gases, monitoring SpO2 and EtCO2 while the gas flow through the ECMO oxygenator is completely off. Due to this success, our physician group has integrated DLCTs into standard management of our high-risk respiratory ECMO cases as we feel this will assist in objective evaluation of lung recovery (or lack thereof) within our care team.
Conclusion
ECMO is a therapeutic modality employed with increased frequency for patients who would have historically been considered sub-optimal candidates. An example is our patient with CF, ARDS, sepsis, inotropic-dependent shock and renal failure. We were able to bridge this patient to recovery using a multifaceted approach, which included a previously unreported way to objectively assess lung recovery while on ECMO. We suggest that using a regular, objective assessment of lung function (namely DLCT) on ECMO can mitigate practice variations and help streamline ECMO weaning, decannulation and prognosis. Furthermore, a standardized assessment tool may aid in improving communication, outcomes and care in challenging ECMO patients.
Conflict of Interest and Role of Funding Source Statements
The authors declare no scientific, financial or personal conflicts of interest. There was no internal or external funding for this manuscript.
Author Contributions
Care and decision making of patient: SA, SSR, AIC, MEN, MRR; Data collection and analysis: SA, BDB, MEN, MRR; Drafting and critical editing of manuscript: SA, BDB, MEN, MRR.
| References | ▴Top |
- Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P,
et al . Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888-1895.
doi pubmed - Noble DW, Peek GJ. Extracorporeal membrane oxygenation for respiratory failure: past, present and future. Anaesthesia. 2010;65(10):971-974.
doi pubmed - Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905-1914.
doi pubmed - Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39(2):364-370.
doi pubmed - Dalton HJ. Extracorporeal life support: moving at the speed of light. Respir Care. 2011;56(9):1445-1453, discuiion 1453-1446.
- Kuhl T, Langebartels G, Madershahian N, Wahlers T. [Extracorporeal life support given to a 16-year-old girl with cystic fibrosis, candida pneumonia and acute respiratory distress syndrome]. Dtsch Med Wochenschr. 2010;135(42):2071-2075.
doi pubmed - Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL,
et al . Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-1363.
doi - Aigner C, Wisser W, Taghavi S, Lang G, Jaksch P, Czyzewski D, Klepetko W. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg. 2007;31(3):468-473, discussion 473-464.
- Lang G, Taghavi S, Aigner C, Renyi-Vamos F, Jaksch P, Augustin V, Nagayama K,
et al . Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation. 2012;93(7):729-736.
doi pubmed - Boussaud V, Mal H, Trinquart L, Thabut G, Danner-Boucher I, Dromer C, Raymond CS,
et al . One-year experience with high-emergency lung transplantation in France. Transplantation. 2012;93(10):1058-1063.
doi pubmed - Nosotti M, Rosso L, Palleschi A, Lissoni A, Crotti S, Marenghi C, Colombo C,
et al . Bridge to lung transplantation by venovenous extracorporeal membrane oxygenation: a lesson learned on the first four cases. Transplant Proc. 2010;42(4):1259-1261.
doi pubmed - Fischer S, Hoeper MM, Tomaszek S, Simon A, Gottlieb J, Welte T, Haverich A,
et al . Bridge to lung transplantation with the extracorporeal membrane ventilator Novalung in the veno-venous mode: the initial Hannover experience. ASAIO J. 2007;53(2):168-170.
doi pubmed - Bermudez CA, Rocha RV, Zaldonis D, Bhama JK, Crespo MM, Shigemura N, Pilewski JM,
et al . Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg. 2011;92(4):1226-1231, discussion 1231-1222. - Hayes D
Jr , Kukreja J, Tobias JD, Ballard HO, Hoopes CW. Ambulatory venovenous extracorporeal respiratory support as a bridge for cystic fibrosis patients to emergent lung transplantation. J Cyst Fibros. 2012;11(1):40-45.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


