| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 4-5, May 2024, pages 67-71
Norepinephrine and Dobutamine-Induced Dynamic Left Ventricular Outflow Tract Obstruction Caused by Systolic Anterior Motion
Tran Duc Hunga, Pham Vu Thu Haa, Do Van Chienb, c
aCardiovascular Center, Military Hospital 103, Vietnam Military Medical University, Hanoi, Vietnam
bDepartment of Acute Cardiac Care, 108 Central Military Hospital, Hanoi, Vietnam
cCorresponding Author: Do Van Chien, Department of Acute Cardiac Care, 108 Central Military Hospital, Hanoi, Vietnam
Manuscript submitted March 19, 2024, accepted April 15, 2024, published online May 2, 2024
Short title: LVOTO Caused by SAM
doi: https://doi.org/10.14740/jmc4204
| Abstract | ▴Top |
This study presents a case of norepinephrine and dobutamine-induced dynamic left ventricular outflow tract obstruction (LVOTO) caused by systolic anterior motion (SAM) in a patient experiencing acute anterior myocardial infarction (MI). In a 76-year-old patient presenting with acute MI, intensive use of norepinephrine and dobutamine may lead to the development of dynamic LVOTO and SAM. The presence of hypotension and a new cardiac murmur may suggest a mechanical complication such as acute mitral regurgitation (MR) or ventricular septal rupture (VSR). The assessment of the left ventricular outflow tract (LVOT) using echocardiography plays a critical role in the diagnosis of SAM and its associated MR and dynamic LVOTO. The patient’s condition was stabilized through the cessation of inotropes and the implementation of aggressive fluid resuscitation, resulting in improved hemodynamics. In conclusion, prompt identification of the underlying pathophysiological mechanisms is imperative for effectively managing this condition and preventing hemodynamic exacerbation.
Keywords: Left ventricular outflow tract obstruction; Systolic anterior motion; Acute myocardial infarction; Norepinephrine; Dobutamine
| Introduction | ▴Top |
Systolic anterior motion (SAM) of the mitral valve is a recognized phenomenon in which the mitral valve leaflet moves in an anterior direction and comes into contact with the left ventricular outflow tract (LVOT) during systole [1], which may occur after LVOT obstruction (LVOTO). Both phenomena are associated with increased mortality [2]. SAM has four grades: grade 0 (non-existing), grade 1 (deviation > 5 mm (M-mode), grade 2 (non-holosystolic contact to ventricular septum) and grade 3 (holosystolic contact to ventricular septum) [3]. SAM may also arise in post-myocardial infarction (MI) patients and patients undergoing mitral valve repair or with adverse risk factors such as hypovolemia or catecholamine overexposure [2].
In cardiogenic shock secondary to acute coronary syndrome, inotropes and vasopressors are used to maintain hemodynamics. Inotropes and vasopressors are also known to induce LVOTO and SAM in various contexts [4-6], along with other factors (e.g., epinephrine [7]). Inotropes can lead to or worsen dynamic LVOTO due to its inotropic and chronotropic effects [8]. Meanwhile, vasopressors stimulate alpha1 and beta1 adrenergic receptors [9], which can result in elevating heart rate, increasing force of contraction in the heart muscles, and narrowing of the arteries and veins, leading to increased preload and afterload [10]. Therefore, norepinephrine is claimed to be less likely to cause or exacerbate LVOTO [11]. This study presents a case of norepinephrine and dobutamine-induced dynamic LVOTO caused by SAM in a patient experiencing acute anterior MI.
| Case Report | ▴Top |
Investigations
A 76-year-old man with hypertension, diabetes, and smoking (20 cigarettes per day for 20 years) was admitted to the hospital with a complaint of less likely cardiac chest pain at home. However, on admission, he denied any chest pain and shortness of breath. Blood pressure was 140/100 mm Hg, and oxygen saturation was normal (98%). There were no heart murmurs during auscultation. Electrocardiography (ECG) showed a sinus heart rate of 66 without ST changes. Transthoracic echocardiography (TTE) indicated normal left ventricular (LV) dimensions (LV end-diastolic dimension index (LVEDDI) = 36 mL/m2 and LV end-systolic dimension index (LVESDI) = 26 mL/m2), 53% left ventricular ejection fraction (LVEF), and normal LV wall motion. There was no evidence of mitral regurgitation (MR) on Doppler imaging. Other echocardiogram findings included concentric LV hypertrophy (septal thickness was 10 and 14 mm, and posterior wall thickness was 11 and 15 mm at diastole and systole, respectively. The estimated LV mass index was 152 g/m2). The patient initially remained hemodynamically stable, and underwent clinical investigation for chest pain.
After 12 h of hospitalization, the patient had chest pain and became unstable with low blood pressure (80/40 mm Hg) and tachycardia (heart rate 120 bpm). Initially, the patient was resuscitated with fluid infusion and a norepinephrine dose of 0.1 µg/kg/min and waited for cathlab activation. During the waiting time, his blood pressure continued to reduce, thus, an additional dobutamine dose of 5 µg/kg/min was used. A new 3/6 holosystolic murmur was heard at the apex, radiating to the axilla and the left sternal border. Results of ECG showed ST elevation in V2-V4 leads that might suspect an acute coronary syndrome. Bedside ECG revealed hyperkinesis with preserved global contractility (LVEF 75.1%). Concentric LV hypertrophy was more pronounced with septal thicknesses of 17.4 and 21.1 mm and posterior wall thicknesses of 16.9 and 17.4 mm in diastole and systole, respectively. Doppler ECG showed the systolic anterior movement of the mitral leaflet with turbulence in the LVEF and severe MR. A “dagger-shaped” continuous wave Doppler pattern characteristic of dynamic LVOT obstruction was observed with a peak velocity of 4.34 m/s and a peak gradient of 75 mm Hg. No mechanical complications of MI were observed during ECG. An apical four-chamber view showed a sigmoid-shaped septum with apical hypokinesia and compensatory hyperkinesia of the basal segments. Aortic, pulmonary and tricuspid valves were normal (Figs. 1, 2).
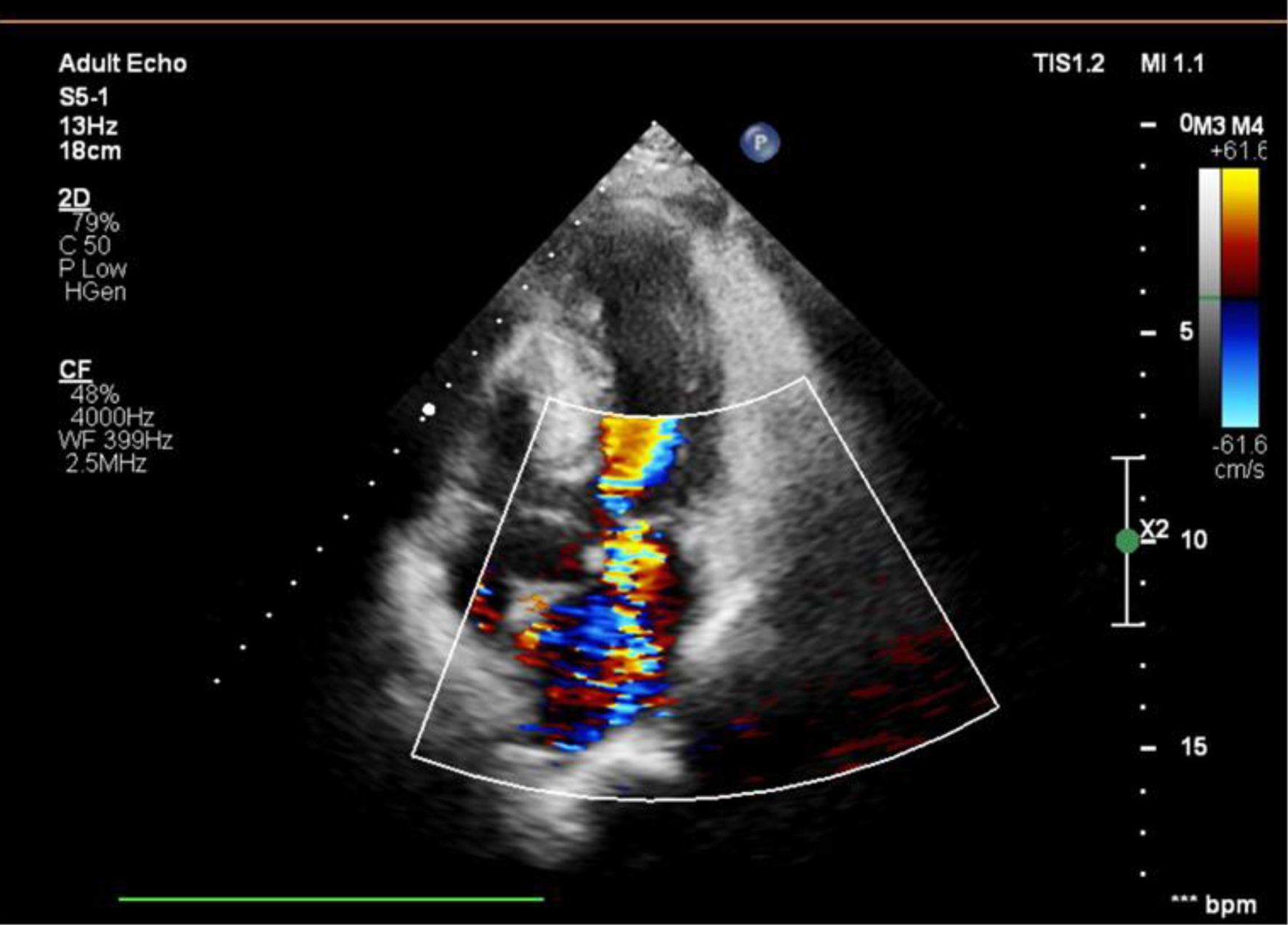 Click for large image | Figure 1. Doppler imaging showing severe MR (MR area 10.4 cm2; vena contracta: 7 mm). MR: mitral regurgitation. |
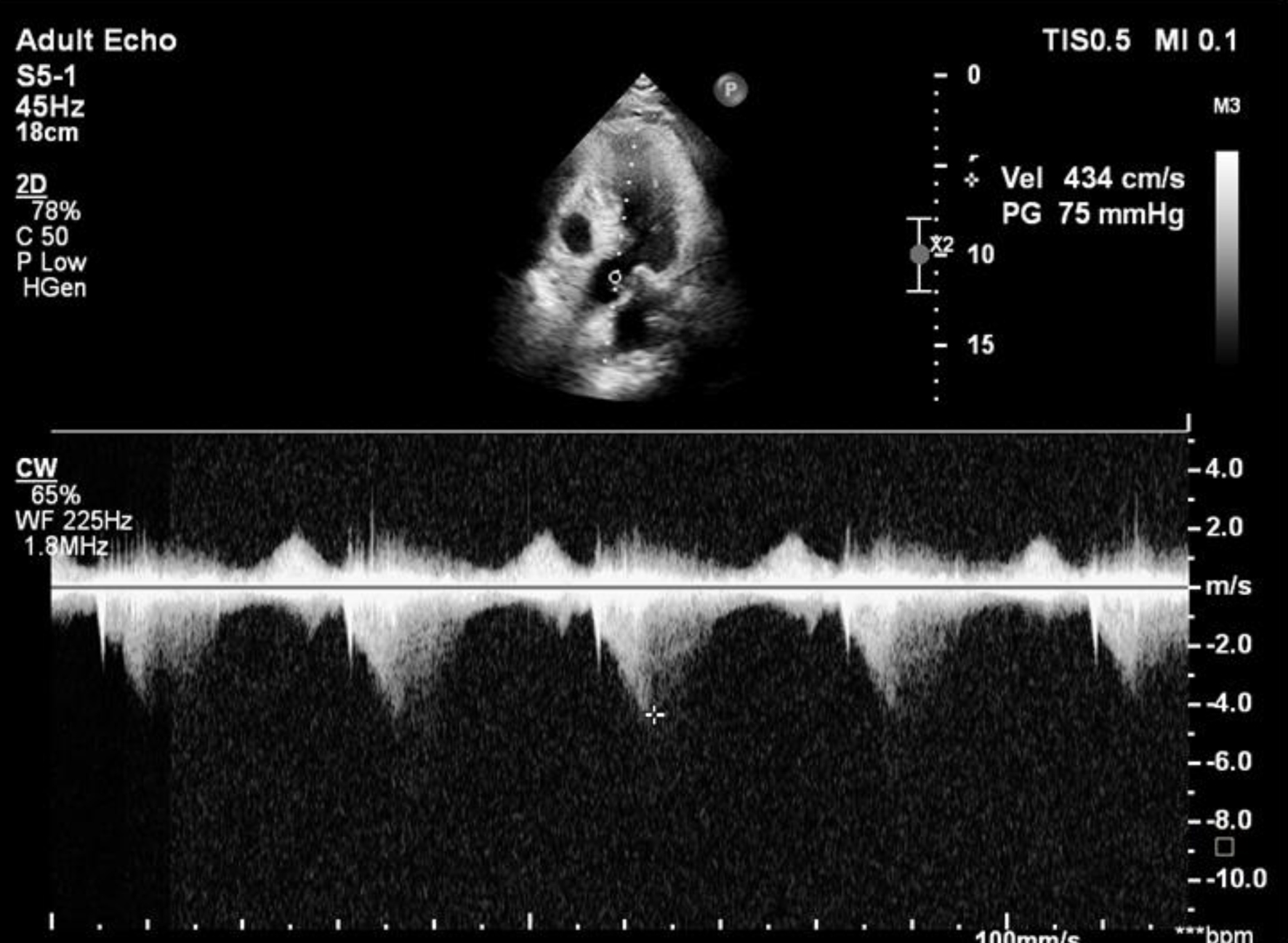 Click for large image | Figure 2. Doppler echocardiography revealed a late peaking high velocity (4.34 m/s) signal in the left ventricular outflow tract. |
Diagnosis
The patient was transferred to the catheterization laboratory, and coronary angiography and percutaneous coronary intervention (PCI) were performed. Coronary angiography showed partial occlusion of the left anterior descending (LAD) artery. A mid-LAD lesion was treated with PCI and drug-eluting stent implantation. However, the patient’s condition did not improve. Considering adequate myocardial contraction with LVEF of 75%, poor hemodynamics could be related to SAM-derived dynamic LVOTO and MR.
Treatment
A decision was made to stabilize the patient by eliminating causes of SAM, e.g., discontinuation of inotropes and aggressive fluid resuscitation, which resulted in hemodynamic improvement. As the causes of SAM were effectively addressed, the systolic murmur disappeared and repeated ECG confirmed normal motion of the anterior mitral leaflet, competent mitral valve and absence of LVOTO.
Follow-up and outcomes
After 3 days, the symptoms of heart failure subsided, and the cardiac murmur disappeared. Hemodynamics returned to normal with blood pressure of 120/80 mm Hg, heart rate of 70 bpm, and SpO2 of 98%. The 24-h urine output was 3,000 mL without diuresis, and central venous pressure (CVP) was 12 mm Hg. ECG results showed that the LVEF was normal (52%), and MR was graded as mild without any evidence of LVOTO. The thickness of the interventricular septum returned to normal, and the regional walls did not show any signs of damage. There was no murmur audible over the heart. The patient was discharged, in a stable condition, to return home. At the 4-week follow-up, the patient had a good recovery, and TTE reexamination confirmed that the patient had excellent LV function and mild MR with no LVOTO.
| Discussion | ▴Top |
SAM was first observed in individuals with asymmetric hypertrophic cardiomyopathy (HCM) in the 1960s and was considered one of the classic symptoms of the disease. HCM is the leading cause of sudden cardiac death, and the implantation of an implantable cardioverter-defibrillator (ICD) is seen as appropriate when patients exhibit any of the following risk factors: a history of sudden death in immediate family members, a maximal LV wall thickness of ≥ 30 mm, or a recent unexplained syncopal event [3, 12]. However, SAM and LVOTO are not only associated with HCM but also occur in many diseases such as elongated MV leaflets [13] and can even be found in individuals with structurally normal hearts [2]. SAM and LVOTO can both develop independently under different clinical conditions, including LV hypertrophy (hypertrophy or sigmoid septum), reduced LV chamber size (due to dehydration, haemorrhage, or diuresis) and mitral valve defects (redundant, long anterior leaflets) and hypercontraction (stress, using inotropes, angiotensin-converting enzyme inhibitor (ACE-I), digitalis or effects of catecholamines) [3, 4]. Acute MI with symptoms of SAM and dynamic LVOTO has also been documented recently [4-6]. Although occasionally discovered during an MI, until now, the MI guidelines have not addressed this phenomenon and its management [14]. The mechanism of dynamic LVOTO appears to be compensatory hyperkinesis of normally perfused residual myocardium just proximal to the coronary occlusion. This hyperkinesis reduces the systolic LVOT cross-sectional area and creates a Venturi effect, which “suctions” the anterior mitral valve leaflet into contact with the septum, causing SAM [6]. LV hypertrophy and a “bulging” subaortic septum are also significant risk factors for SAM [14].
In our case, ECG showed that the LV was concentrically hypertrophic, the septum had a sigmoidal shape, and there was compensatory hyperkinesia with basal segments. A well-established phenomenon is hyperactive contractility of myocardial segments close to an acutely ischemic zone. This mechanism is most likely to occur in patients with single-vessel disease of the LAD artery, while multivessel disease may prevent basal hyperkinesis [6, 14]. The pathogenesis of LVOTO in patients with severe coronary artery stenosis leading to myocardial ischemia has been related to the anterior apical motion abnormality and increased hyperkinesis seen at LV basal areas. LVOTO may also be connected to a hyperdynamic state induced by inotropes. Inotrope agents are often used to maintain systemic blood pressure but may cause positive chronotropy in cardiac shock. However, dobutamine aggravates dynamic LVOTO in this group of patients [15].
The diagnosis of dynamic LVOTO is critical because the treatment of cardiac shock without an understanding of the underlying pathophysiology of this condition may worsen the patient’s hemodynamics, i.e., inotropic agents may increase the LV chamber-aorta pressure gradient, which worsen SAM and lead to more severe LVOTO and MR [16]. Using inotropes in a volume-depleted and small LV may provoke transient SAM [17]. In contrast, sufficient volume loading increases the LV size and the LVOT diameter [18]. In our patient, he might have less likely cardiac chest pain, causing doctors to be subjective and not pay attention to the diagnosis of MI. Only after 12 h of hospitalization, when the patient complained chest pain and hypotension (raised with noradrenalin and dobutamine), the murmur was heard. After that, the ECG results showed ST elevation, and the echocardiography results (due to the murmur) diagnosed LVOTO and SAM. There was no murmur during norepinephrine infusion. We supposed that the cause of hypotension was low volume state and started intravenous (IV) fluid infusion. Systolic murmur occurred only after adding an IV infusion of dobutamine. A new murmur and hypotension might frequently be misdiagnosed with mechanical complications of MI, i.e., acute MR due to ruptured chordae or ventricular septal rupture (VSR). Bedside ECG is an efficient tool to differentiate these conditions. Our bedside ECG showed severe MR and LVOTO due to SAM, which may be triggered by hypovolemia and excessive inotropes in this patient, as both increase transaortic flow velocity [18] and are responsible for severe LVOTO in this case. Determination of dynamic LVOTO allowed treatment to be optimized on a case-by-case basis. Martinez-Useros et al reported a case of severe LAD stenosis resulting in dynamic LVOTO, which was subsequently resolved by coronary angioplasty to reduce initial hypercontractility [4]. However, in the patient described in the present report, hypovolemia and an inotropy-related hyperdynamic state may be important mechanisms causing severe LVOTO, as discontinuation of dobutamine along with echocardiography-guided parenteral fluid and CVP resulted in the rapid improvement of hemodynamics within hours. In the case of reanimation in HCM patients, phenylephrine is the best option [19].
Patients with acute MI might develop a dynamic pressure gradient in the LVOT. This is an interesting new finding that carries therapeutic implications. If the pathophysiological mechanism is recognized early, it may help doctors prevent heart attacks from occurring in the first place. LVOTO was reported to cause cardiac rupture in patients with acute coronary syndrome [5]. The clinical consequences of asynchronous LVOTO may result from insufficient cardiac output. Furthermore, it leads to increased end-systolic wall stress in infarcted segments and thereby plays a part in the pathogenesis of myocardial rupture [5]. It is essential to make a precise initial diagnosis of these patients. This helps uncover potentially curable underlying illnesses such as acute heart failure, acute MR, dynamic LVOTO and myocardial rupture. This case report demonstrates that hypovolemia combined with an inotrope-associated hyperdynamic state can cause dynamic LVOTO in acute MI even with normal cardiac function. Early diagnosis and timely management of these major risk factors for SAM are critical to managing this condition.
In conclusion, in a patient with acute MI, intensive use of norepinephrine and dobutamine may cause dynamic LVOTO and SAM. The presence of hypotension and a new cardiac murmur may suggest the development of a mechanical complication, i.e. acute MR or VSR. However, it may be simply due to SAM associated with acute anterior MI and inappropriate management. Echocardiographic evaluation of the LVOT is essential to diagnose SAM and its hemodynamic consequences of MR and dynamic LVOTO. Early recognition of the underlying pathophysiological mechanisms is necessary for the effective management of this condition and for the avoidance of hemodynamic exacerbation.
Learning points
Norepinephrine and dobutamine in acute MI patients may lead to dynamic LVOTO with SAM, worsening hemodynamics.
Auscultation and bedside echocardiography can help identify the cause of hemodynamic compromise and new murmur in acute MI.
Echocardiography is essential for diagnosing SAM and its hemodynamic consequences, including MR and dynamic LVOTO.
Mechanisms of the shock should be carefully considered when starting treatment.
Acknowledgments
The authors would like to thank the patient for providing data.
Financial Disclosure
This study received no funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The patient has given consent to participate and to publish the data.
Author Contributions
Tran Duc Hung and Pham Vu Thu Ha conceptualized and collected data; Do Van Chien validated data. All authors prepared, edited and revised the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Maron BJ, Epstein SE. Hypertrophic cardiomyopathy. Recent observations regarding the specificity of three hallmarks of the disease: asymmetric septal hypertrophy, septal disorganization and systolic anterior motion of the anterior mitral leaflet. Am J Cardiol. 1980;45(1):141-154.
doi pubmed - Ibrahim M, Rao C, Ashrafian H, Chaudhry U, Darzi A, Athanasiou T. Modern management of systolic anterior motion of the mitral valve. Eur J Cardiothorac Surg. 2012;41(6):1260-1270.
doi pubmed - Sakellaropoulos SG, Steinberg BS. Hypertrophic cardiomyopathy: a cardiovascular challenge becoming a contemporary treatable disease. Cardiol Res. 2023;14(4):243-249.
doi pubmed pmc - Chockalingam A, Tejwani L, Aggarwal K, Dellsperger KC. Dynamic left ventricular outflow tract obstruction in acute myocardial infarction with shock: cause, effect, and coincidence. Circulation. 2007;116(5):e110-e113.
doi pubmed - Bartunek J, Vanderheyden M, de Bruyne B. Dynamic left ventricular outflow tract obstruction after anterior myocardial infarction. A potential mechanism of myocardial rupture. Eur Heart J. 1995;16(10):1439-1442.
doi pubmed - Haley JH, Sinak LJ, Tajik AJ, Ommen SR, Oh JK. Dynamic left ventricular outflow tract obstruction in acute coronary syndromes: an important cause of new systolic murmur and cardiogenic shock. Mayo Clin Proc. 1999;74(9):901-906.
doi pubmed - Nooli NP, Jensen MA, Lawson P, Jr., Tuck BC, Sipe SS, Nanda NC, Townsley M. Role of rescue transesophageal echocardiography during intraoperative anaphylaxis complicated by dynamic left ventricular outflow tract obstruction. J Cardiothorac Vasc Anesth. 2023;37(4):565-569.
doi pubmed - Aurigemma G, Battista S, Orsinelli D, Sweeney A, Pape L, Cuenoud H. Abnormal left ventricular intracavitary flow acceleration in patients undergoing aortic valve replacement for aortic stenosis. A marker for high postoperative morbidity and mortality. Circulation. 1992;86(3):926-936.
doi pubmed - Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24(5):293-316.
doi pubmed - Krejci V, Hiltebrand LB, Sigurdsson GH. Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis. Crit Care Med. 2006;34(5):1456-1463.
doi pubmed - Monnet X, Jabot J, Maizel J, Richard C, Teboul JL. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med. 2011;39(4):689-694.
doi pubmed - Trivedi A, Knight BP. ICD therapy for primary prevention in hypertrophic cardiomyopathy. Arrhythm Electrophysiol Rev. 2016;5(3):188-196.
doi pubmed pmc - Sakellaropoulos S, Svab S, Mohammed M, Dimitra L, Mitsis A. The role of mitral valve in hypertrophic obstructive cardiomyopathy: an updated review. Curr Probl Cardiol. 2021;46(3):100641.
doi pubmed - Hrovatin E, Piazza R, Pavan D, Mimo R, Macor F, Dall'Aglio V, Burelli C, et al. Dynamic left ventricular outflow tract obstruction in the setting of acute anterior myocardial infarction: a serious and potentially fatal complication? Echocardiography. 2002;19(6):449-455.
doi pubmed - Auer J, Berent R, Weber T, Lamm G, Eber B. Catecholamine therapy inducing dynamic left ventricular outflow tract obstruction. Int J Cardiol. 2005;101(2):325-328.
doi pubmed - Conradi PM, van Loon RB, Handoko ML. Dynamic left ventricular outflow tract obstruction in Takotsubo cardiomyopathy resulting in cardiogenic shock. BMJ Case Rep. 2021;14(3):e240010.
doi pubmed pmc - Abbas H, Senthil Kumaran S, Zain MA, Ahmad A, Ali Z. Transient systolic anterior motion of the anterior mitral valve leaflet in a critical care patient with a structurally normal heart. Cureus. 2019;11(1):e3963.
doi pubmed pmc - Manabe S, Kasegawa H, Arai H, Takanashi S. Management of systolic anterior motion of the mitral valve: a mechanism-based approach. Gen Thorac Cardiovasc Surg. 2018;66(7):379-389.
doi pubmed - Moreno JD, Bach RG, Damiano RJ, Martinez SC, Cresci S. Phenylephrine provocation to evaluate the cause of mitral regurgitation in patients with obstructive hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2021;14(5):e012656.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


