| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 7, July 2023, pages 260-264
Disseminated Histoplasmosis in a Patient With Acquired Immunodeficiency Syndrome in a Non-Endemic Region (California)
Alexander T. Phana, d , Ankur Bhagata, Bahareh Maknounib, Momin Masroorc, Mufadda Hasanb
aDepartment of Internal Medicine, Arrowhead Regional Medical Center, Colton, CA 92324, USA
bDepartment of Critical Care Medicine, Arrowhead Regional Medical Center, Colton, CA 92324, USA
cCalifornia University of Science and Medicine, Colton, CA 92324, USA
dCorresponding Author: Alexander T. Phan, Department of Internal Medicine, Arrowhead Regional Medical Center, Colton, CA 92324, USA
Manuscript submitted May 24, 2023, accepted July 3, 2023, published online July 31, 2023
Short title: Disseminated Histoplasmosis in California
doi: https://doi.org/10.14740/jmc4097
| Abstract | ▴Top |
Histoplasmosis is caused by infection with Histoplasma capsulatum (H. capsulatum). Progressive disseminated histoplasmosis is a more severe form of histoplasmosis and is seldom diagnosed in non-endemic regions of the world owing to the fungus’s geographical distribution. In the United States (USA), Histoplasma capsulatum is classically known to be endemic to the Mississippi and Ohio River valleys, and cases in non-endemic areas, such as the southwest USA, are exceedingly rare. Patients with acquired immunodeficiency syndrome (AIDS) are at risk for infection with H. capsulatum, and failure to recognize and treat histoplasmosis may be devastating to patients. In non-endemic regions, the proposed mechanism for disseminated histoplasmosis in AIDS patients is reactivation of a previous infection. Here, we present the case of a young male patient who presented to a southern California hospital with diarrhea, was diagnosed with AIDS, and developed acute hypoxic respiratory failure. Chest imaging revealed diffuse reticulonodular opacities, and histoplasmosis was confirmed by urine and serologic examination. He was subsequently treated with liposomal amphotericin B and safely discharged from the hospital with oral itraconazole therapy. This case contributes to the current limited body of literature citing histoplasmosis infections in California, and clinicians should consider histoplasmosis as a differential diagnosis in non-endemic regions.
Keywords: Disseminated histoplasmosis; Infectious disease; Pulmonology; Acquired immunodeficiency syndrome; Human immunodeficiency virus; Immunocompromised
| Introduction | ▴Top |
Histoplasma capsulatum (H. capsulatum) is a dimorphic fungus that causes an endemic mycosis and is classically known to be endemic to the Mississippi and Ohio River valleys of the United States [1, 2]. Of the endemic mycoses, it is the most common cause of hospitalization and death in the United States [2]. The clinical spectrum of this disease is broad and can be classified based on disease cadence (acute, subacute, chronic), onset (primary versus reactivation), distribution (pulmonary, mediastinal, disseminated, extrapulmonary), and severity (asymptomatic, mild, moderate, severe) [1]. The geographic distribution of histoplasmosis is poorly understood; however, several hypothesized factors for increasing incidence include moderate temperatures and bird or bat guano exposure [1, 2].
Patients in non-endemic regions may also experience histoplasmosis. The most common reason for histoplasmosis in non-endemic regions is prior exposure to H. capsulatum in an endemic region [3]. Another modality for developing non-endemic histoplasmosis is reactivation of an old infection, which has been noted in patients with acquired immunodeficiency syndrome (AIDS) [3]. Patient risk factors for developing histoplasmosis include extremes of age, infection with human immunodeficiency virus (HIV), AIDS, solid organ transplant, bone marrow transplant, and use of immunosuppressive medications [1, 2].
In particular, nearly all patients infected by HIV, or those who have progressed to AIDS, develop progressive disseminated histoplasmosis (PDH) due to primary infection with H. capsulatum [1]. PDH develops when the inhaled yeast overcomes the host’s immunological defense mechanisms and spreads through the blood, seeding to other organs such as the liver, spleen, gastrointestinal tract, central nervous system, skin, lungs, and adrenal glands [1, 2]. Thus, due to the propensity for multiorgan involvement in PDH, prompt treatment is necessary, as is consultation with an infectious disease expert. Here, we describe the case of a 33-year-old male who presented to a California hospital, developed acute hypoxic respiratory failure, and was ultimately diagnosed with PDH based on urinary testing and bronchoscopy pathologic findings.
| Case Report | ▴Top |
Investigations
A 33-year-old male with no significant past medical history presented to our emergency department with chronic diarrhea of 1 month’s duration, odynophagia, and abdominal pain. He reported three to four episodes of non-bloody diarrhea daily, non-bloody, non-bilious emesis, yellow-colored sputum production, cough, fevers, chills, and dyspnea. He denied any hematochezia, melena, mucoid stools, rash, weight loss, night sweats, sick contacts, previous hospitalizations, or previous surgeries. He also denied active tobacco, alcohol, or illicit drug use. His family history was unknown, as he had been estranged from his family. At the time of presentation, he worked in a taco restaurant in San Bernardino County, California and denied any recent travel outside of the county. Regarding occupational exposures, he denied ever working in a shipyard, with industrial chemicals, in a factory, with jet fuel, or on a farm. He also denied having pets or exploring caves. Sexual history was notable for multiple male sexual partners while living in Michoacan, Mexico, where he was born and raised. The patient’s initial vital signs demonstrated a temperature of 38.3 °C, pulse rate of 124, respiratory rate of 18, blood pressure of 111/62 mm Hg, and oxygen saturation of 97% on ambient air. Physical exam revealed an ill-appearing, fatigued young male with mild, non-tender cervical lymphadenopathy and diffuse abdominal tenderness to deep palpation. He did not have any rashes or skin lesions, and his lungs were clear to auscultation bilaterally without increased work of breathing.
Diagnosis
His initial blood counts and metabolic panel were notable for a normocytic anemia, mild bandemia, moderate hyponatremia, mild hypokalemia, and mild hypochloremia (Table 1). Serology was positive for human immunodeficiency virus (HIV) screening. Severe acute respiratory syndrome virus 2 (SARS-CoV-2) polymerase chain reaction (PCR) testing was negative. Blood, sputum, and stool cultures were also obtained at this time. Computed tomography (CT) of the abdomen and pelvis without intravenous (IV) contrast was unremarkable, except for mild splenomegaly.
 Click to view | Table 1. Initial Laboratory Findings, Demonstrating Anemia, Hyponatremia, Hypokalemia, and Hypochloremia |
The patient was started on IV ceftriaxone and metronidazole therapy for sepsis due to a likely intra-abdominal source. Over the course of the next 24 - 48 h, the patient began complaining of worsening shortness of breath requiring supplemental low flow oxygen. A chest X-ray revealed diffuse bilateral reticulonodular opacities (Fig. 1). Due to a concern for atypical pneumonia, IV azithromycin was added to his antibiotic regimen. Shortly thereafter, he continued to desaturate on 5 L of nasal cannula supplemental oxygen, so he was placed on high flow nasal cannula with fraction of inspired oxygen (FiO2) of 35% and flow rate of 60 L/min. Due to a concern for pulmonary embolism, CT angiogram of his chest was obtained, demonstrating patent pulmonary arteries; however, diffuse ground glass opacifications, mediastinal adenopathy, and hilar adenopathy were noted (Fig. 2). All the while, the patient continued to have a productive cough and low-grade fevers. The diagnostic workup is detailed in Figure 3.
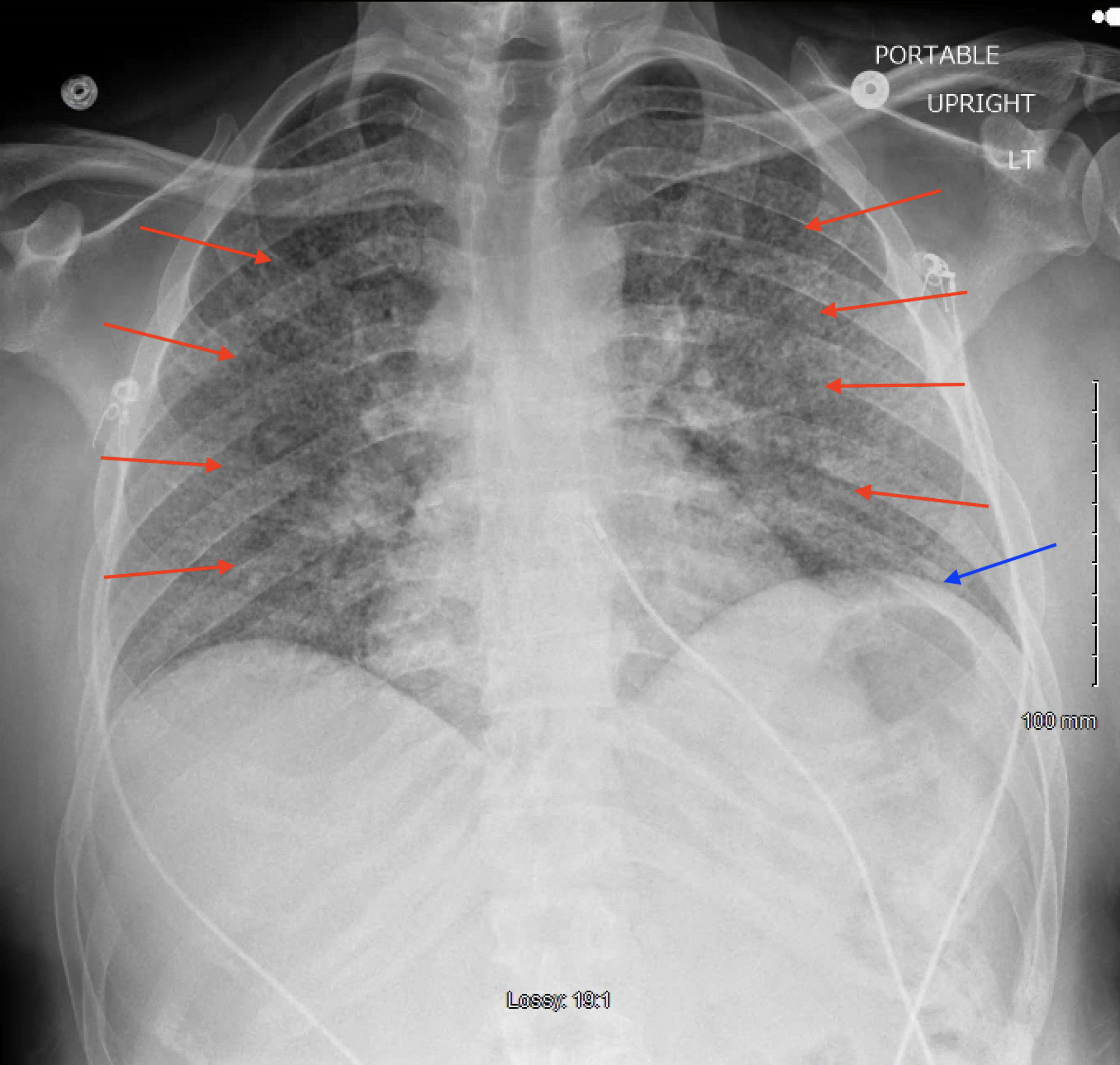 Click for large image | Figure 1. Posterior-anterior chest radiograph demonstrating diffuse bilateral reticulonodular opacifications (red arrows) and elevated left hemidiaphragm (blue arrow). |
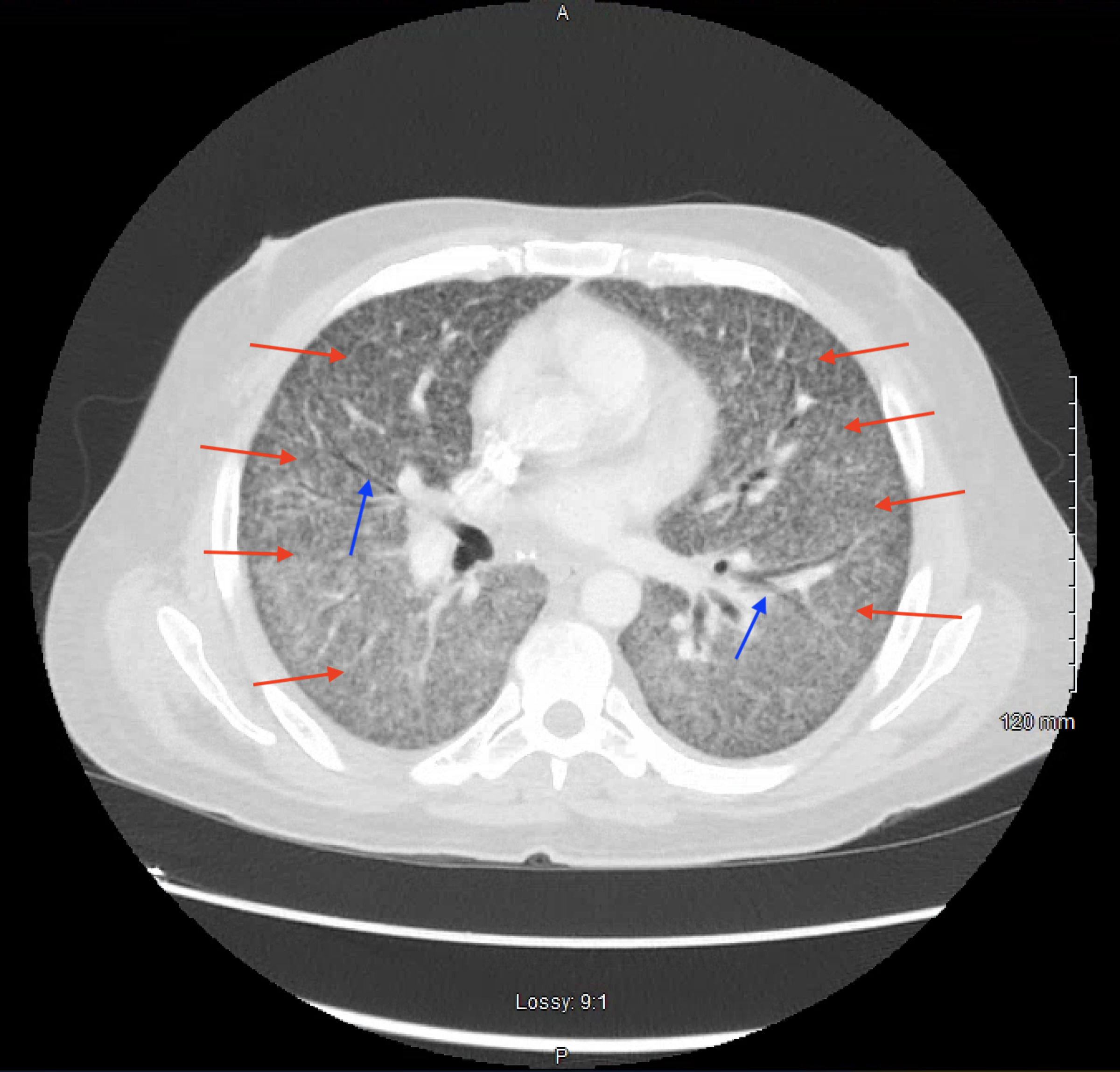 Click for large image | Figure 2. Axial view of a computed tomography of the chest with intravenous contrast demonstrating diffuse reticulonodular opacities (red arrows), ground glass opacities, and air bronchograms (blue arrows). |
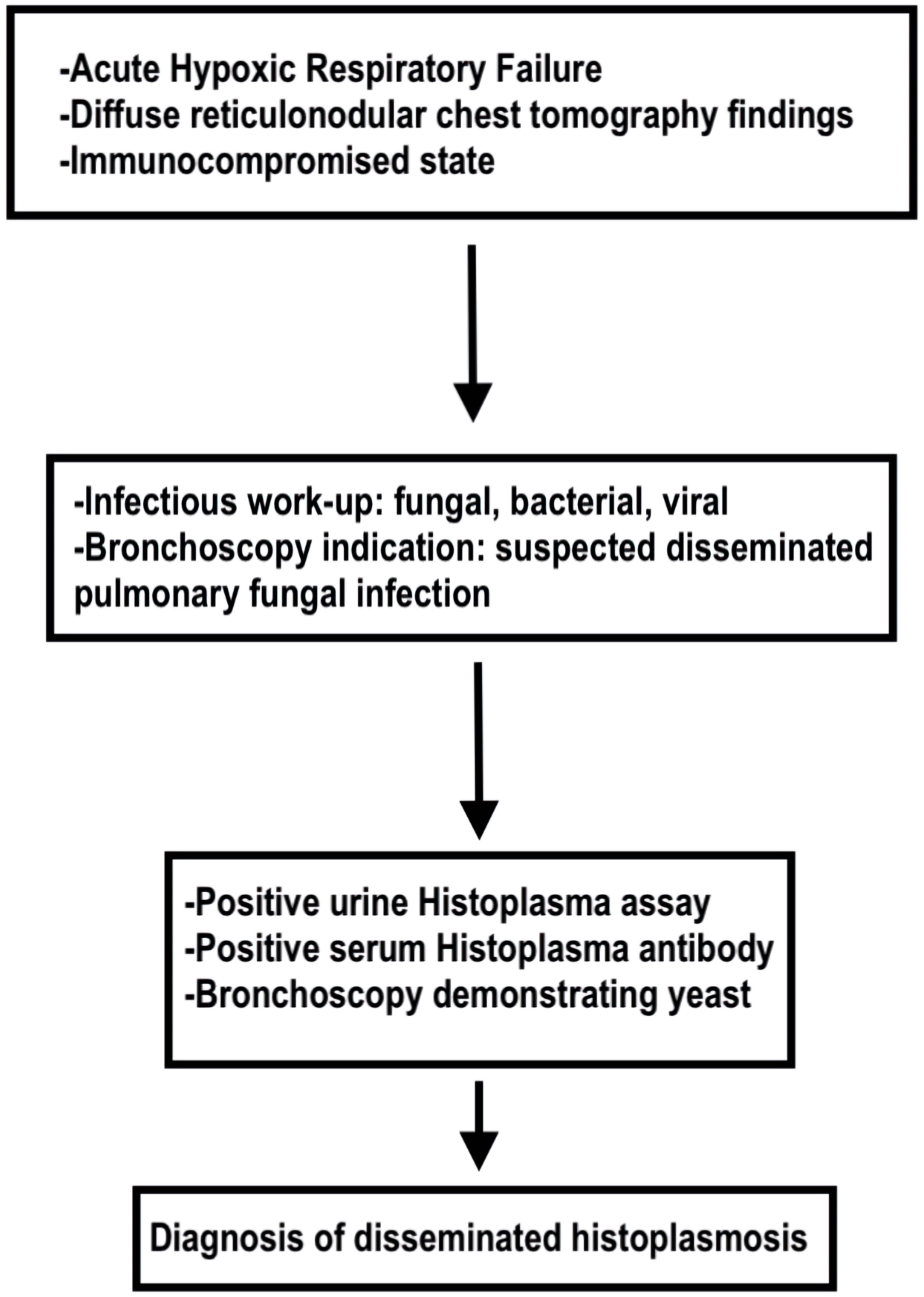 Click for large image | Figure 3. Flow diagram of diagnostic workup for disseminated histoplasmosis. |
The next day, the patient’s absolute CD4 cell count was noted to be severely low at < 20 (n = 490 - 1,740 cells/µL) and serum 1,3-beta-D-glucan was positive. With the input of the Infectious Disease specialist, he was started on IV trimethoprim-sulfamethoxazole 600 mg every 6 h and oral prednisone 40 mg daily due to concern for Pneumocystis jiroveci pneumonia (PJP) in the setting of an AIDS diagnosis. Regarding cultures, blood and stool cultures did not demonstrate growth; however, sputum cultures demonstrated growth of methicillin-sensitive Staphylococcus aureus and Enterobacter cloacae. Three separate sputum acid-fast bacilli (AFB) smears were negative, ruling out active tuberculosis. Clostridium difficile toxin PCR, Epstein-Barr virus quantitative real-time PCR (RT-PCR), and cytomegalovirus RT-PCR, Toxoplasma immunoglobulin (Ig)M and IgG, urine Streptococcus pneumoniae antigen, urine Legionella antigen, serum Coccidioides antibody, and serum cryptococcal antigen testing were all negative. Celiac disease was also ruled out based on serologic testing. Interestingly, the patient was noted to have serum Histoplasma antibody complement fixation titers of 1:32 (n ≤ 1:8) and positive results for urine Histoplasma galactomannan enzyme immunoassay (MiraVista Diagnostics), suggesting a diagnosis of disseminated histoplasmosis.
The patient subsequently underwent a flexible bronchoscopy with bronchoalveolar lavage (BAL) for further characterization of pulmonary findings. During the procedure, the vocal cords, trachea, and upper airways were within normal limits; however, thin, frothy, white secretions were appreciated in the right and left lungs, which were sent for fluid studies. Results were negative for AFB smear, AFB culture, and Pneumocystis jirovecii direct fluorescent assay. Respiratory cultures from the bronchoscopy yielded rare yeast, and cytology from the BAL revealed scattered foamy macrophages containing clusters of tiny intracytoplasmic yeasts, suggesting pulmonary histoplasmosis. Left lower lobe lingular washings also revealed intracytoplasmic organisms which may have represented Histoplasma as well.
Treatment
In the setting of this patient’s disseminated histoplasmosis, the Infectious Disease and Pulmonary specialists recommended starting treatment with IV liposomal amphotericin B 3 mg/kg/day for 2 weeks in addition to adjunctive treatment with IV methylprednisolone 60 mg daily. Highly active antiretroviral therapy (HAART) was initiated with daily oral bictegravir/emtricitabine/tenofovir. Treatment for PJP was discontinued, and prophylaxis for PJP and toxoplasmosis was initiated with oral atovaquone 1,500 mg daily. Additionally, due to positive sputum cultures suggesting a superimposed bacterial infection, as mentioned above, IV cefepime 2 g every 8 h was administered for a 7-day course.
Our patient’s symptoms slowly began to improve over the course of the hospital stay with targeted therapy for disseminated histoplasmosis, AIDS, and a superimposed bacterial pneumonia. After 1 week of this targeted therapy, the patient’s supplemental oxygen requirements decreased, and he was eventually completely weaned off oxygen support. After 2 weeks of amphotericin B, the patient was transitioned to oral itraconazole 300 mg (three times a day for 3 total days), followed by oral itraconazole 200 mg two times a day until outpatient follow-up in the Infectious Disease clinic. The patient was also transitioned to oral steroids with a tapering schedule set in place.
Follow-up and outcomes
At the time of discharge, 3 weeks after presentation to the hospital, the patient’s symptoms had all resolved and he felt that he was back to his baseline. He was scheduled for strict outpatient follow-up in our institution’s Primary Care clinic, Infectious Disease clinic, and Pulmonology clinic. He has followed up in the aforementioned clinics and tolerated his treatment well, without any adverse events.
| Discussion | ▴Top |
Though histoplasmosis is endemic to the Mississippi and Ohio River valleys, it may also present in non-endemic regions due to a variety of patient factors. Benedict et al (2016) identified 105 outbreaks of histoplasmosis, defined by at least two cases of histoplasmosis associated with a common environmental source, in the United States between 1938 and 2013, none of which occurred in California [4]. Further, in the last decade, only one case report has described histoplasmosis in California [5]. Thus, a diagnosis of histoplasmosis in California is exceedingly rare. Through a detailed travel history, we identified that the likely source of initial infection for our patient occurred while living in Michoacan, Mexico, where the patient was born and grew up. The patient immigrated to the United States 7 months prior to presentation. Thus, reactivation of H. capsulatum is the likely reason for his current disseminated infection, consistent with the mechanism for non-endemic disease manifestation in AIDS patients described by Wheat (2006) [3].
The diagnosis of histoplasmosis is typically multifaceted, including histopathological examination, culture, antigen detection, and antibody detection [5]. In a large multicenter study, urine Histoplasma antigen testing had a sensitivity of 91% and a specificity of 99% [6]. They also demonstrated that, in cases of proven PDH, cultures were positive 91.6% of the time, pathology was positive 85.3% of the time, and anti-Histoplasma antibodies were positive 73.6% of the time [6]. Based on these results, a broad approach to diagnosing histoplasmosis is supported [6]. In our case, the patient’s laboratory testing was significant for positive urine antigen testing and serum antibody testing, suggesting a diagnosis of PDH, as evidence of Histoplasma was found separately in the blood and urine. Additionally, diffuse pulmonary reticulonodular opacifications noted on thoracic imaging, typical of PDH, and a history of untreated AIDS further corroborates this diagnosis [1, 2].
Based on the 2007 Infectious Disease Society of America (IDSA) guidelines, the treatment of moderately severe and severe PDH typically includes daily IV liposomal amphotericin B (3 mg/kg) for 1 - 2 weeks followed by oral itraconazole (200 mg three times daily, followed by 200 mg twice daily) for at least 12 months [1-3, 7]. Mild-to-moderate PDH may be treated with oral itraconazole monotherapy for at least 12 months [7]. Additionally, for patients who are unable to reverse immunosuppression, lifelong itraconazole therapy may be warranted [7]. Our patient received 2 weeks of liposomal amphotericin B followed by oral itraconazole therapy, which is consistent with the current IDSA guidelines. He did not experience any adverse effects from medical therapy and was discharged with close follow-up to ensure compliance with histoplasmosis treatment and antiretroviral therapy.
This case report’s strength lies in the fact that the patient underwent a thorough pulmonary and overall infectious disease workup. The case is limited in that there are such few cases of PDH in California; however, we believe that this limitation allows us to contribute to the limited current body of literature citing cases of PDH in California.
Conclusions
PDH is a rare diagnosis in non-endemic regions of the United States such as California. AIDS patients with histoplasmosis present with a myriad of nonspecific infectious symptoms, and failure to consider histoplasmosis in the differential diagnosis may lead to delays in care for this vulnerable population. Consequently, it is important for clinicians to recognize manifestations of non-endemic mycoses and obtain a full infectious workup in immunocompromised patients, as histoplasmosis is a treatable condition.
Learning points
Physicians in areas not endemic to histoplasmosis may not consider this at the top of their differential diagnoses, leading to delays in diagnosis and treatment. As global travel is easily accessible to many, physicians should treat all immunocompromised patients with a broad scope of infectious diseases, including non-endemic mycoses. It is important for all healthcare providers to become familiar with the manifestations and diagnosis of histoplasmosis to promote prompt treatment of a potentially life-threatening disease.
Acknowledgments
The authors would like to thank and express their gratitude to Arrowhead Regional Medical Center’s exceptional nursing staff and unit managers for their expert clinical support.
Financial Disclosure
The authors have no financial or funding disclosures.
Conflict of Interest
The authors declare there is no conflict of interest.
Informed Consent
Informed consent was obtained from the family of the patient. The patient was also appropriately de-identified for this manuscript.
Author Contributions
AP, AB, BM and MM contributed to the initial manuscript write-up, literature review, and editing of the manuscript. MH attended on the case and contributed to decision-making, management of the patient, literature review, and editing of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AIDS: acquired immunodeficiency syndrome; H. capsulatum: Histoplasma capsulatum; USA: United States; CT: computed tomography; PDH: progressive disseminated histoplasmosis; HIV: human immunodeficiency virus; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; PCR: polymerase chain reaction; RT-PCR: real-time polymerase chain reaction; AFB: acid-fast bacilli; BUN: blood urea nitrogen; IV: intravenous; FiO2: fraction of inspired oxygen; PJP: Pneumocystis jiroveci pneumonia; HAART: highly active antiretroviral therapy
| References | ▴Top |
- Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
doi pubmed - Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016;30(1):207-227.
doi pubmed - Wheat LJ. Histoplasmosis: a review for clinicians from non-endemic areas. Mycoses. 2006;49(4):274-282.
doi pubmed - Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938-2013. Emerg Infect Dis. 2016;22(3):370-378.
doi pubmed pmc - Choi J, Nikoomanesh K, Uppal J, Wang S. Progressive disseminated histoplasmosis with concomitant disseminated nontuberculous mycobacterial infection in a patient with AIDS from a nonendemic region (California). BMC Pulm Med. 2019;19(1):46.
doi pubmed pmc - Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, Hammoud K, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53(5):448-454.
doi pubmed - Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


