| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 4, April 2023, pages 130-136
An Uncommon Presentation of Vasopressin-Induced Purpura Fulminans
Vanessa Awada, e, Preeth Naira, Sasmit Royb, e, Anish Yalamanchilic, Sreedhar Adapad, Nirupama Vemuria
aDepartment of Internal Medicine, Sierra View Medical Center, Porterville, CA, USA
bDepartment of Internal Medicine, University of Virginia, Charlottesville, VA, USA
cDepartment of Medicine, Albany Medical College, Albany, NE, USA
dDepartment of Internal Medicine, Adventist Health, Hanford, CA, USA
eCorresponding Author: Sasmit Roy, Department of Internal Medicine, University of Virginia, Lynchburg Dialysis Unit, Lynchburg, VA 24501, USA; Vanessa Awad, Department of Internal Medicine, Sierra View Medical Center, Porterville, CA 93257, USA
Manuscript submitted February 17, 2023, accepted April 13, 2023, published online April 30, 2023
Short title: Uncommon Vasopressin-Induced Purpura Fulminans
doi: https://doi.org/10.14740/jmc4062
| Abstract | ▴Top |
Purpura fulminans (PF) is a rarely encountered rapidly evolving dermatological manifestation of ischemia, particularly in critically ill patients. Considered one of the very few dermatological emergencies, it has high mortality rate where patients often succumb to the illness. It can manifest in three forms: neonatal, idiopathic, and the more commonly infectious variety, which can be secondary to mostly bacterial and rarely viral etiology. It is also reported to be highly associated with disseminated intravascular coagulation (DIC), heparin-induced thrombocytopenia (HIT), and acute hepatic failure (AHF). Hereditary or acquired deficiency of protein C and dysregulation of the coagulation cascade, mainly protein C-thrombomodulin, has been implicated in the pathogenesis. We present a 55-year-old male admitted to the intensive care unit for diabetic ketoacidosis (DKA) and septic shock. Along with initiating management protocol for DKA and broad-spectrum antibiotics, he was initially started on norepinephrine for septic shock. Because of persistent refractory septic shock, he was subsequently initiated on phenylephrine and vasopressin to maintain adequate perfusion. The following day, he was found to have sharply demarcated blackish non-blanching discoloration on bilateral knees, lower limbs, and scrotum, sparing the acral regions. This cutaneous manifestation persisted throughout his hospital course, although it improved after discontinuation of vasopressin while continuing with other pressors. Vasopressin has been implicated in a few instances of skin necrosis; however, PF has rarely been documented and never within 1 day like ours. This case demonstrates a unique development of PF likely from vasopressin after ruling out the diagnoses of DIC, HIT, thrombotic thrombocytopenic purpura, and AHF.
Keywords: Vasopressin; Purpura fulminans; Systemic peripheral gangrene; Disseminated intravascular coagulation; Thrombocytopenia
| Introduction | ▴Top |
Purpura fulminans (PF) is a rare cutaneous disease characterized by an acute purpuric rash, most commonly precipitated by increased intravascular coagulation.
It is an infrequently encountered, rapidly evolving dermatological manifestation of ischemia, particularly in critically ill patients. Considered one of the very few dermatological emergencies, it comes with a high mortality rate where patients often succumb to the illness from thrombosis [1]. It was first described in 1884 by Guelliot in children [1]. It can manifest in three forms: neonatal, idiopathic, and the more common infectious variety, which can be secondary to mostly bacterial, and rarely viral, etiology [2].
Vasopressin is a vital stress hormone released internally in cases of low perfusion state like shock [3]. It is considered a second-line pressor agent in critically ill patients where perfusion is essential to maintain life. Dermatological side effects are rarely reported and are mostly limited to skin necrosis [4, 5].
Here we describe a unique case of PF induced by vasopressin with resolution of skin manifestations after discontinuation of the inciting agent.
| Case Report | ▴Top |
Investigations
A 55-year-old male with a past medical history of insulin-dependent diabetes mellitus (unknown type) and methamphetamine abuse (inhalation, intravenous) presented to the emergency department (ED) with altered mentation. He was found to be tachycardic, tachypneic, and hypothermic in the ED, with a Glasgow coma scale (GCS) of 10. There was documented use of recent methamphetamine use. On the initial physical exam, he was noted to be a disheveled man, obtunded, and confused. The rest of the systemic physical examination was unremarkable. Labs on admission are shown in Table 1. Notable findings were elevated leucocytes, increased lactic acid, increased procalcitonin, increased anion gap, severely decreased bicarbonate levels, and elevated serum creatinine.
 Click to view | Table 1. Initial Laboratory Values on Admission |
Diagnosis
Meeting 4/4 systemic inflammatory response syndrome (SIRS) criteria, he was started on intravenous (IV) fluids as per sepsis protocol and initiated on broad-spectrum antibiotics-vancomycin and zosyn. Diabetic ketoacidosis (DKA) protocol was commenced with IV fluids and IV insulin. Subcutaneous heparin was initiated as prophylaxis for deep vein thrombosis.
Initially, on admission, his lowest recorded blood pressure (BP) was 96/62 mm Hg with mean arterial pressure (MAP) of 73 mm Hg. Because of increasing respiratory demand in the background of encephalopathy, he was intubated the following morning. He became hypotensive after intubation with an MAP of 52 mm Hg and was started on norepinephrine for blood pressure support. Later that afternoon, vasopressin and phenylephrine were added due to increasing norepinephrine requirement with persistently low MAP. Phenylephrine was discontinued later that night, while vasopressin was continued until the next day. When the patient was reexamined in the morning, he was found to have sharply demarcated non-blanching purpura on the base of the penis, diffusely over the scrotum, bilateral knees, bilateral anterior distal lower limbs, with an area of necrosis in the right lateral knee, measuring approximately 1 × 1 cm (Fig. 1a-d).
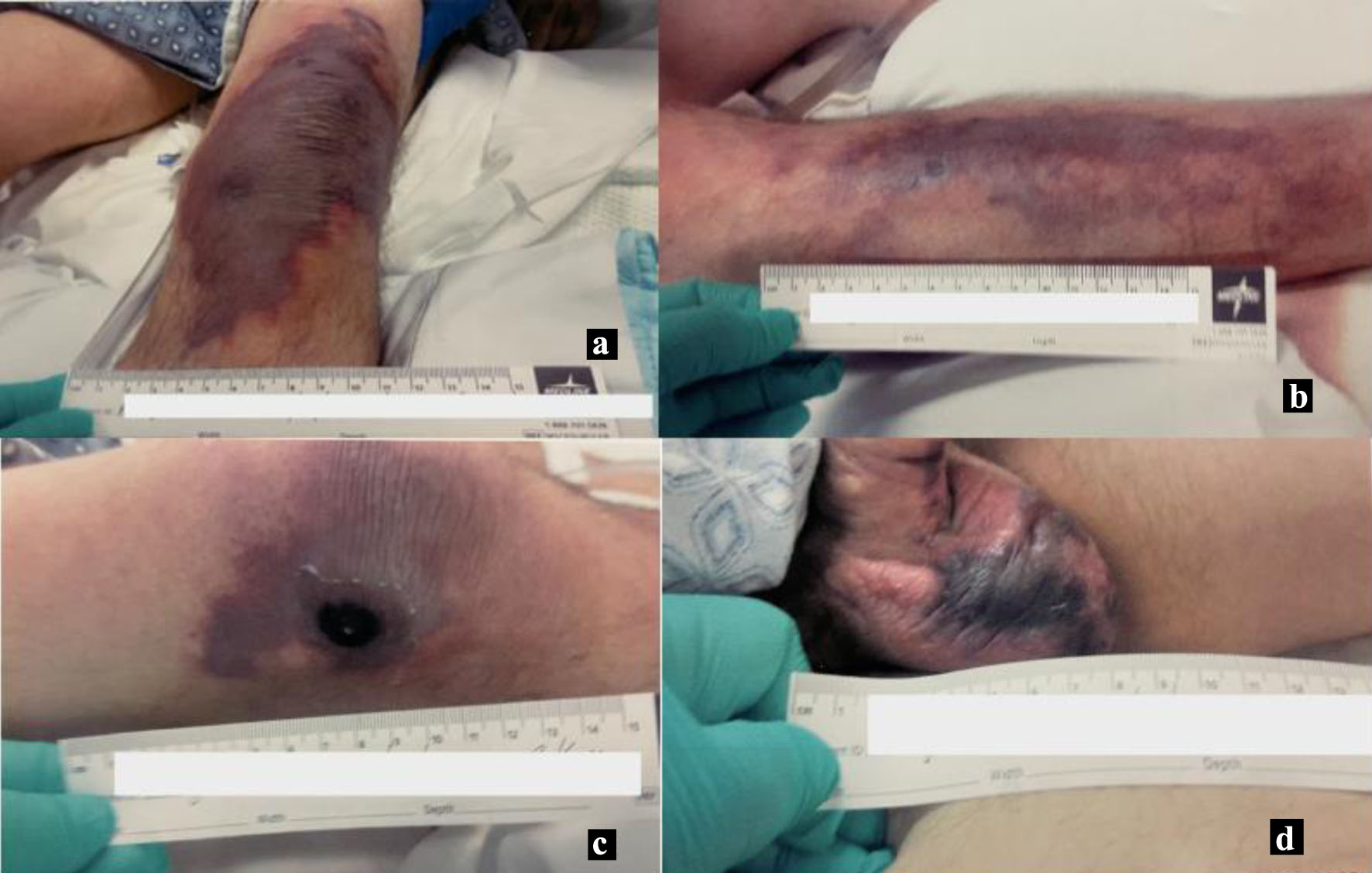 Click for large image | Figure 1. Skin findings the morning following initial vasopressin use. (a) Left lower leg. (b) Right lower leg. (c) Right lateral knee. (d) Scrotum. |
Treatment
Labs on the next morning are shown in Table 2. There was a significant drop in platelet count with persistently elevated white blood cell (WBC) count with improving lactic acid values, anion gap, and serum creatinine. Blood cultures came back positive for Klebsiella pneumonia and antibiotics were tapered down to zosyn alone. Heparin was held and disseminated intravascular coagulation (DIC) and heparin-induced thrombocytopenia (HIT) panels were sent. Pressor use thereafter ranged from norepinephrine, and phenylephrine, to no pressors, as his day-to-day MAP was labile. While awaiting HIT and DIC results, platelets count continued to drop daily, from 74,000 to 52,000, to 31,000, and to 12,000 within the following 48 h.
 Click to view | Table 2. Laboratory Values on the Second Day of Hospitalization |
A peripheral smear stained with Wright’s stain was sent. It showed normocytic normochromic anemia with anisocytosis, including schistocytes (accounting for approximately 1% of the red blood cells), nucleated red blood cells, severe thrombocytopenia, and mature neutrophilia with unremarkable morphology (Fig. 2).
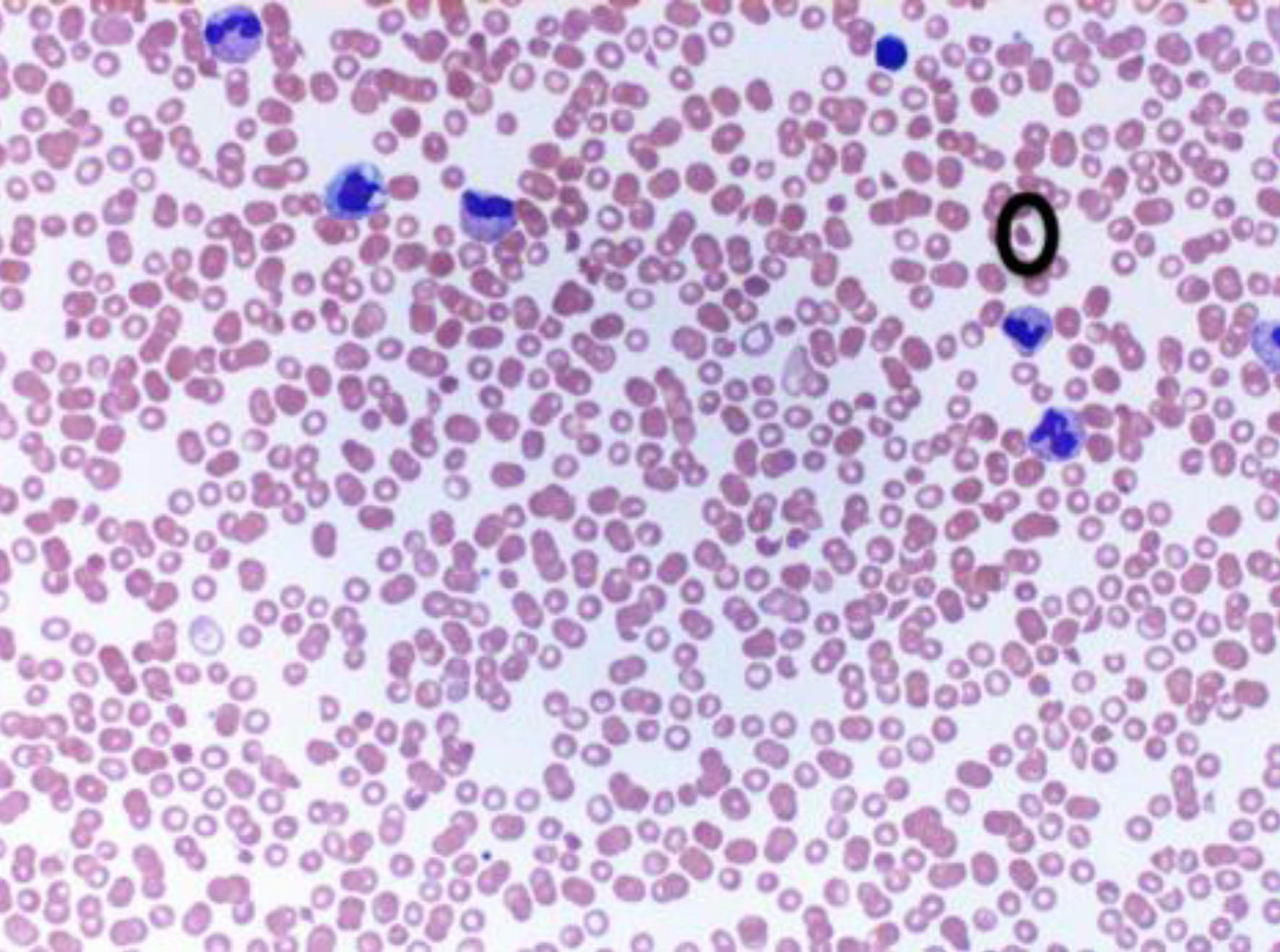 Click for large image | Figure 2. Peripheral blood smear with schistocyte marked. |
After consulting hematology, lactate dehydrogenase (LDH) and ADAMTS13 levels were also obtained as thrombotic thrombocytopenic purpura (TTP) was considered as a possibility (Table 3). The haptoglobin level was normal and there was no elevated bilirubin level which argued against the possibility of hemolytic anemia and overall TTP was considered unlikely.
 Click to view | Table 3. Laboratory Values |
Follow-up and outcomes
Over the subsequent week, the patient’s need for vasopressors subsided, and most of the purpura, although not entirely resolved, had significantly improved, as shown in Figure 3a-d. One important thing to mention here, all throughout this time he did not require vasopressin anymore and for pressor support was only put on norepinephrine as needed. His labs also got significantly better with near normalization of platelets, WBC, and creatinine levels. His condition improved throughout hospitalization, and he was subsequently discharged to long-term rehabilitation unit. After being discharged from the rehab, he has been lost to follow-up as he never showed up for his subsequent outpatient clinic visits.
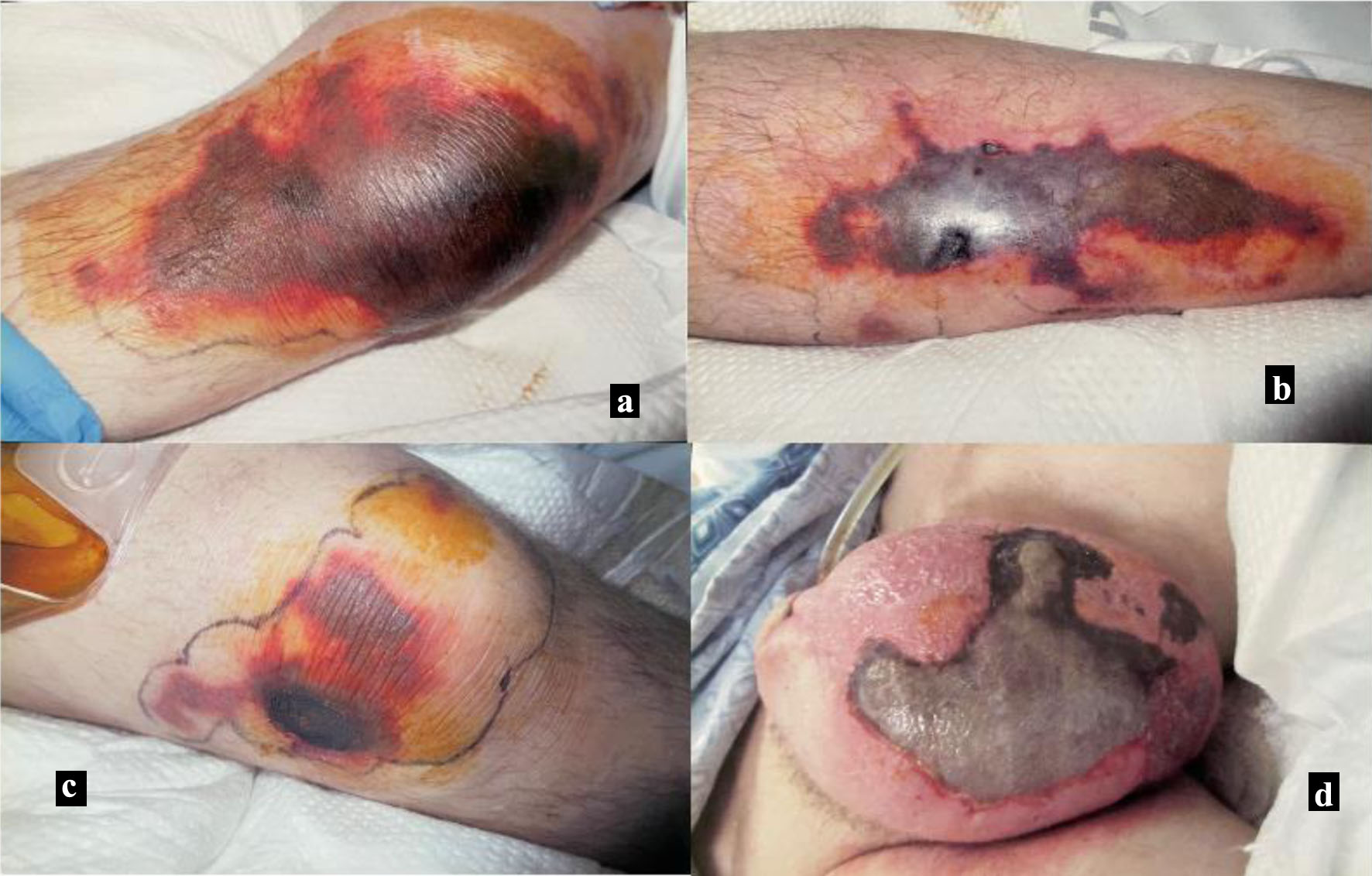 Click for large image | Figure 3. Skin findings 9 days after initial vasopressin use. (a) Left lower leg. (b) Right lower leg. (c) Right lateral knee. (d) Scrotum. |
| Discussion | ▴Top |
PF, or symmetrical peripheral gangrene, is a rarely encountered manifestation of acute necrosis, defined as ischemia at two or more sites in multiple extremities in the absence of significant vessel obstruction [6, 7]. As it is a rarely encountered disease, articles describing this diagnosis are commonly case reports or case series [3]. It may manifest in three forms: neonatal (a hereditary deficiency of anticoagulants), idiopathic (a postulated post-infectious autoimmune disorder), and acute infectious (seen in severe acute sepsis) [3]. In addition, DIC, HIT, and acute hepatic failure (AHF) have been reported to be the most prevalent associated causes of this rare cutaneous phenomenon [8]. Few medications have been implicated in literature precipitating PF as well, which include phenytoin, quinine, diclofenac, ketorolac, and seldom, vasopressin [9].
Vasopressor use in the setting of shock, regardless of shock type, is a mainstay of therapy to achieve hemodynamic stability in critically ill patients. The most used agents for critically ill shock patients include norepinephrine, dopamine, vasopressin, and epinephrine, with their use varying on the type of shock present. Septic shock is a subdivision of distributive shock wherein a vast systemic inflammatory response leads to widespread vascular endothelial damage and altered vascular tone, causing extravasation of intravascular contents into peripheral tissues and extracellular matrix, despite adequate fluid resuscitation. First-line therapy for such shock includes tailored antibiotic therapy and vasopressor support, particularly with norepinephrine [10]. When septic shock becomes refractory, the addition of second-line agents, such as vasopressin, is required to maintain an adequate MAP [10]. Although these agents have been used as lifesaving supportive measures, they are not without adversity: ischemia of mesenteric and renal circulation, decreased cardiac index, thrombocytopenia with preserved coagulation and elevated liver function tests. Several pressors have been reported in the literature to have effects in splanchnic circulation and, more remarkably, the peripheral systemic circulation [4, 11]. The following discussion dives into the differential diagnoses of PF, an incredible phenomenon, and by a process of elimination, unravels vasopressin as the culprit at hand.
Initially, TTP was thought to be the diagnosis of such skin manifestations in our patients. TTP is caused by deficiency in the ADAMTS13 protein, a von Willebrand factor (vWF) cleaving protease [12]. Without this protease, these large vWF multimers cannot be cleaved, causing platelet binding and forming microthrombi [13]. The ADAMTS13 level obtained was 0.52 IU/mL, slightly below the lower end of normal. Normally, activity below 0.10 IU/mL is seen in acquired and hereditary TTP. Also, there were no features of microscopic hemolytic anemia which is common in TTP. As a result, further inhibitor assays were not performed since the established diagnosis was not met, arguing against this differential as the etiology for the above clinical scenario.
DIC is a clinical and laboratory diagnosis using a few different scoring systems that have been validated. The Japanese Association of Acute Medicine (JAAM)-DIC score is sensitive to predicting DIC in the setting of sepsis (with a score of ≥ 4 supporting a diagnosis of DIC). Our patient’s score was 4/12, arguing against a conclusive diagnosis. However, fibrinogen levels would be low in acute DIC as it becomes consumed. In our patient, fibrinogen levels were significantly elevated and remained elevated, thereby eliminating DIC as a cause of the current scenario [10]. In a retrospective study by Siegal et al, they reported that the main clinical manifestations seen in DIC of increasing frequency are bleeding, renal dysfunction, liver dysfunction, respiratory dysfunction, shock, thromboembolic phenomena, and central nervous system (CNS) involvement. Although our patient exhibited renal dysfunction and shock, these manifestations preceded the cutaneous manifestation of PF, making DIC less likely [14].
HIT, defined as a ≥ 50% decrease in platelet count post heparin exposure, can occur in one of two forms: type I or non-immune and type II or immune-mediated. The former typically develops 5 - 10 days after heparin exposure, whereas the latter can occur in 24 - 48 h due to preformed antibodies from previous exposure to heparin [15, 16]. Diagnostic testing for either form includes obtaining assays for platelet-factor 4 with the strength of reaction correlating to the probability of disease (IgG-specific enzyme-immunoassay (EIA) > 1.0 OD units) [16]. Our patient not only had a value of < 1.0, but serum tests were also negative for heparin-induced platelet antibodies, and the lesions occurred in less than 12-h period. Thrombocytopenia, in this case, could have been induced by ongoing severe sepsis.
AHF, also known as “shock liver”, is another precipitant of PF. In critically ill patients, AHF is commonly caused by hypoperfusion from severe hypotension and loss of vascular tone. As the synthetic function of the liver is lost in AHF, this leads to dysfunction of coagulation proteins [17]. Additionally, liver function tests like aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) are expected to be significantly elevated, with AST and ALT in the thousands [18]. The patient presented here had elevated ALP and mildly elevated AST on admission, which all had resolved, excluding AHF as a predisposing factor for PF.
Among infectious causes, bacteremia is most implicated in etiology of PF. Meningococcus species-related infection is considered the most common cause [19]. Other common bacteria that cause this illness are Hemophilus influenza, Staphylococcus aureus, Streptococcus species, and Capnocytophaga canimorsus [20].
PF has been considered a subtype of DIC, which is highly pro-thrombotic [21]. The pathophysiology of this condition still needs to be better understood. However, endothelial cell breakdown, along with the unregulated thrombomodulin-protein C system, is considered the root cause of this hypercoagulable state [20]. Abnormal protein C and its associated proteins are the common denominators in PF-related pathology. It has been observed that there is a substantial decrease in circulating antithrombin, protein C, and protein S which are important innate components of the thrombotic pathway. The widely cited article by Faust et al [22] discovered that endothelial protein C receptor and endothelial thrombomodulin level expression was much lower in meningococcal sepsis-related PF patients compared to controls.
Treatment is primarily supportive and aimed at the underlying cause, especially infectious etiology. IV hydration and anticoagulation are beneficial in its management [5]. In infectious etiology like our case, broad-spectrum antibiotics covering Neisseria, Staphylococcus, and Streptococcus are preferred. IV immunoglobulin has also been used as antibodies to these toxins [23]. Activated protein C has also been attempted to help with the coagulation pathway but with mixed results and is not recommended presently [20]. Therapeutic plasma exchange with fresh frozen plasma has also been tried, and there are no guidelines for it [20]. Lastly, repeated debridement of affected areas is required based on the clinical scenario [5]. In cases of PF induced by vasopressin, the medicine needs to be stopped like in our case, and other types of pressors need to be commenced.
Learning points
This review provides supplementary updates regarding one of the uncommon etiologies of an already established rare disease not frequently encountered. The aim of the case and discussion presented is meant to guide clinicians who encounter this cutaneous disease towards other etiologies should the common culprits (DIC, HIT, TTP, and AHF) be ruled out. However, further studies are of great interest and are needed to deepen our understanding of said critical illness dermatopathy.
Conclusion
Vasopressor use has been frequently documented to cause severe peripheral constriction, notably with norepinephrine’s effects in the distal phalanges. However, very few reports with vasopressin were published, noting necrosis at the extravasation sites or the muscular regions of the limb, as in the case reported herein [24]. As the conditions intensely and frequently associated with PF had all been ruled out, particularly DIC, it is evident that the culprit at hand had been the addition of vasopressin, a unique development causing a rare cutaneous disease in such a short span of use. It is essential to keep this rare yet potentially life-threatening manifestation in mind in critically ill patients so that recognition and early reversal of the underlying cause may be addressed.
Acknowledgments
None to declare.
Funding Disclosure
None to disclose.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
The patient consented for publication of this study and the written consent form is available to authors for submission if requested.
Author Contributions
Each author has been individually involved in and has made substantial contributions to conceptions and designs, acquisition of data, analysis, interpretation of data, drafting, and editing the manuscript. Vanessa Awad contributed to the acquisition of data, drafting, and editing of the manuscript. Sasmit Roy contributed to the designs, analysis, interpretation of data, and editing of the manuscript and with final submission. Preeth Nair contributed to acquisition of data and editing of the manuscript; Anish Yalamanchili contributed to the designs, interpretation of data and editing of the manuscript; Sreedhar Adapa contributed to the designs, acquisition of data, analysis, interpretation of data, drafting, and editing of the manuscript. Nirupama Vemuri contributed to the drafting and editing of the manuscript.
Data Availability
The data supporting the findings of this study can be accessed via PubMed, Goggle Scholar databases.
| References | ▴Top |
- Betrosian AP, Berlet T, Agarwal B. Purpura fulminans in sepsis. Am J Med Sci. 2006;332(6):339-345.
doi pubmed - Holmes CL, Walley KR. Vasopressin in the ICU. Curr Opin Crit Care. 2004;10(6):442-448.
doi pubmed - Perera TB, Murphy-Lavoie HM. Purpura Fulminans. In: StatPearls. Treasure Island (FL). 2023.
pubmed pmc - Dunser MW, Mayr AJ, Tur A, Pajk W, Barbara F, Knotzer H, Ulmer H, et al. Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med. 2003;31(5):1394-1398.
doi pubmed - Kim EH, Lee SH, Byun SW, Kang HS, Koo DH, Park HG, Hong SB. Skin necrosis after a low-dose vasopressin infusion through a central venous catheter for treating septic shock. Korean J Intern Med. 2006;21(4):287-290.
doi pubmed pmc - Ruffin N, Vasa CV, Breakstone S, Axman W. Symmetrical peripheral gangrene of bilateral feet and unilateral hand after administration of vasopressors during septic shock. BMJ Case Rep. 2018;2018:bcr2017223602.
doi pubmed pmc - Shenoy R, Agarwal N, Goneppanavar U, Shenoy A, Sharma A. Symmetrical peripheral gangrene-a case report and brief review. Indian J Surg. 2013;75(Suppl 1):163-165.
doi pubmed pmc - Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014;2(1):15.
doi pubmed pmc - Kosaraju N, Korrapati V, Thomas A, James BR. Adult purpura fulminans associated with non-steroidal anti-inflammatory drug use. J Postgrad Med. 2011;57(2):145-146.
doi pubmed - Levy JH, Ghadimi K, Faraoni D, van Diepen S, Levy B, Hotchkiss R, Connors JM, et al. Ischemic limb necrosis in septic shock: What is the role of high-dose vasopressor therapy? J Thromb Haemost. 2019;17(11):1973-1978.
doi pubmed - Jain G, Chandran P, Patnaik I, Patel NB. Terlipressin-induced skin necrosis. BMJ Case Rep. 2021;14(11):e246678.
doi pubmed pmc - Chiasakul T, Cuker A. Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology Am Soc Hematol Educ Program. 2018;2018(1):530-538.
doi pubmed pmc - Kremer Hovinga JA, Coppo P, Lammle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020.
doi pubmed - Siegal T, Seligsohn U, Aghai E, Modan M. Clinical and laboratory aspects of disseminated intravascular coagulation (DIC): a study of 118 cases. Thromb Haemost. 1978;39(1):122-134.
pubmed - Baroletti SA, Goldhaber SZ. Heparin-induced thrombocytopenia. Circulation. 2006;114(8):e355-356.
doi pubmed - Greinacher A. Heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(Suppl 1):9-12.
doi pubmed - Warkentin TE, Pai M. Shock, acute disseminated intravascular coagulation, and microvascular thrombosis: is 'shock liver' the unrecognized provocateur of ischemic limb necrosis? J Thromb Haemost. 2016;14(2):231-235.
doi pubmed - McDowell Torres D, Stevens RD, Gurakar A. Acute liver failure: a management challenge for the practicing gastroenterologist. Gastroenterol Hepatol (N Y). 2010;6(7):444-450.
pubmed pmc - Bollero D, Stella M, Gangemi EN, Spaziante L, Nuzzo J, Sigaudo G, Enrichens F. Purpura fulminans in meningococcal septicaemia in an adult: a case report. Ann Burns Fire Disasters. 2010;23(1):43-47.
pubmed pmc - Colling ME, Bendapudi PK. Purpura Fulminans: Mechanism and Management of Dysregulated Hemostasis. Transfus Med Rev. 2018;32(2):69-76.
doi pubmed - Dempfle CE. Coagulopathy of sepsis. Thromb Haemost. 2004;91(2):213-224.
doi pubmed - Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, Laszik Z, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345(6):408-416.
doi pubmed - Edlich RF, Cross CL, Dahlstrom JJ, Long WB, 3rd. Modern concepts of the diagnosis and treatment of purpura fulminans. J Environ Pathol Toxicol Oncol. 2008;27(3):191-196.
doi pubmed - Arnaiz-Garcia ME, Arnaiz-Garcia AM, Gutierrez-Diez JF, Gonzalez-Santos JM, Garcia-Martin A, Alonso-Pena D, Arnaiz J. Vasopressor-induced peripheral skin necrosis after shock. Rev Port Cardiol. 2017;36(7-8):573-574.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


