| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 10, October 2022, pages 499-503
Isolated Renal Relapse in a Post-Allogenic Transplant Adult Patient With Acute Lymphoblastic Leukemia
Marco Alejandro Jimenez-Ochoaa, c , Maria Margarita Contreras-Serratosa, Martha Leticia Gonzalez-Bautistaa, Constantino Lopez-Maciasb
, Diego Alberto Lozano-Jaramilloa
aInstituto Mexicano del Seguro Social, Centro Medico Nacional Siglo XXI, Hospital de Especialidades, Unidad de Trasplante de Medula Osea, Mexico City, Mexico
bInstituto Mexicano del Seguro Social, Centro Medico Nacional Siglo XXI, Hospital de Especialidades, Unidad de Investigacion Medica en Inmunoquimica, Mexico City, Mexico
cCorresponding Author: Marco Alejandro Jimenez-Ochoa, Instituto Mexicano del Seguro Social, Centro Medico Nacional Siglo XXI, Hospital de Especialidades, Unidad de Trasplante de Medula Osea, Mexico City, Mexico
Manuscript submitted September 9, 2022, accepted October 18, 2022, published online October 31, 2022
Short title: ALL Isolated Renal Relapse
doi: https://doi.org/10.14740/jmc4003
| Abstract | ▴Top |
Acute lymphoblastic leukemia (ALL) is an aggressive hematological neoplasm typically more common in children than adults. More prolonged remissions and a potential cure can be achieved if allogeneic hematopoietic stem cell transplantation (allo-HSCT) is performed. Outcomes after allo-HSCT vary significantly among patients, and multiple factors contribute to these outcomes. Isolated extramedullary relapse (iEMR) after allo-HSCT is rare. We present the case of a 43-year-old man who was diagnosed with Philadelphia chromosome-negative (Ph-neg), B-cell ALL and underwent haploidentical allo-HSCT because of high-risk features at diagnosis. One year later, he was admitted to the hospital with facial and peripheral edema, proteinuria, elevated serum creatinine levels, and hypertension. Renal biopsy was performed immediately. Renal infiltration of TdT+ leukemic cells was detected by immunohistochemistry. Bone marrow aspiration, lumbar puncture, and computed tomography (CT) scans were performed to identify other sites of possible relapse. No other sites were identified, and an extramedullary isolated renal relapse was diagnosed. Intensive re-induction with chemotherapy was not possible because of the coronavirus disease 2019 (COVID-19) infection. Six weeks later, a medullary relapse was noted. Medullary infiltration of B-cell ALL after allo-HSCT has a historically poor prognosis; however, iEMR appears to have a better overall prognosis. The optimal treatment for renal iEMR is still a matter of debate.
Keywords: Acute lymphoblastic leukemia; Allogenic hematopoietic stem cell transplantation; Isolated extramedullary relapse
| Introduction | ▴Top |
Philadelphia chromosome-negative (Ph-neg) acute lymphoblastic leukemia (ALL) in adults is an aggressive hematological neoplasm [1]. It is the second most common acute leukemia in adults after acute myeloid leukemia (AML) [2]. As it is today, with chemotherapy, complete remission (CR) can be achieved in approximately 90% of patients [3]. With chemotherapy as the only treatment, long-term survival is achieved in 50% to 70% of patients with Ph-neg precursor B-cell ALL [4]. The decision to proceed with allogeneic hematopoietic stem cell transplantation (allo-HSCT) depends on several prognostic features. The main objective to do so is to maintain more prolonged remissions and achieve a possible oncological cure [4-6]. Relapse after allo-HSCT is estimated at 35%, with a median of 136 days after transplantation in a large single-center study [7]. At diagnosis, the extramedullary involvement rate in ALL is estimated to be 10% [8]. Isolated extramedullary relapse (iEMR) post-allo-HSCT is rare but seems to have a better overall prognosis [9, 10]. Isolated relapse of renal parenchyma after allo-HSCT seems to be quite rare. The incidence, prognosis, and treatment are not well defined [11, 12].
We present a case of a patient with Ph-neg B-cell ALL in whom allo-HSCT was performed successfully about a year prior to presenting with sudden flank pain, edema of the extremities, and foamy urine. In this case presentation, we discuss the diagnosis, follow-up, and treatment options of a patient with B-cell ALL isolated renal parenchyma relapse after allo-HSCT.
| Case Report | ▴Top |
Investigations
A 43-year-old man was referred to our center with a history of bruising, asthenia, and weight loss > 10 kg over 6 months. At diagnosis, laboratory features were as follows: hemoglobin 9.4 g/dL, white blood cells 14 × 103/µL, and platelets 15 × 103/µL. Bone marrow aspirate was ordered and showed 90% lymphoblasts. The immunophenotypic pattern of expression was compatible with B-common ALL (CD19+ CD34+ CD10+ TDT+ CD20-). Cytogenetic studies revealed a complex karyotype with 42 XY, -14, -16, del(17) -19, -22. The final diagnosis of high-risk Ph-neg B-cell ALL was concluded, and treatment with a Berlin-Frankfurt-Munich (BFM)-like protocol was administered. After a month of treatment, he achieved CR and was administered two more consolidation cycles following the same protocol. He was sent for early stem cell transplantation consultation. Allo-HSCT was performed with a familiar haploidentical donor, his 11-year-old daughter, using a myeloablative conditioning regimen (including busulfan, fludarabine, and cyclophosphamide) with graft-versus-host disease (GVHD) prophylaxis based on post-transplant cyclophosphamide on days +3 and +4, mycophenolate from days +5 to +35 and cyclosporine tapering beyond 6 months after transplantation. The dose of CD34 cells was 8 × 106 per kg in the recipient. Myeloid and platelet engraftment was observed on day +14. On day +60, the patient was diagnosed with cutaneous acute GVHD grade I-II and treated successfully with topical steroids.
On day +310 after HSCT, the patient started with mild flank pain responsive to low dose of non-steroidal anti-inflammatory drug (NSAID), palpebral and bilateral lower extremity edema, accompanied by intermittent foamy urine, and he did not refer hematuria or any other symptoms. Physical examination was normal except for swelling in both lower limbs. He was admitted to hospital for the diagnosis procedures and diuretic treatment.
Diagnosis
Laboratory features included hemoglobin of 13.6 g/dL, total leucocyte count of 4.7 × 109/L, platelet count of 323 × 103/µL, lactate dehydrogenase (LDH) level of 211 U/L reported in normal ranges, a creatinine of 3.51 mg/dL, urea concentration of 141 mg/dL, and a 24-h urine protein collection of 1 g. Ultrasound and computed tomography (CT) scans revealed nephromegaly (Fig. 1). Percutaneous kidney biopsy was performed without complications. The pathology department reported seven glomeruli, with kidney parenchyma substituted with infiltrative monotonous cells with middle size suspecting relapsed leukemia; immunohistochemical staining was negative for IgA, IgM, IgG, C1q, C3c, C4c, albumin and light chains (kappa, lambda), but diffuse TdT positivity in the malignant cells (Fig. 2) was reported, concluding renal infiltration by ALL. Extension studies with bone marrow aspirate, minimal residual disease, and lumbar puncture were reported negative for infiltration of ALL. The diagnosis of renal iEMR relapse was concluded.
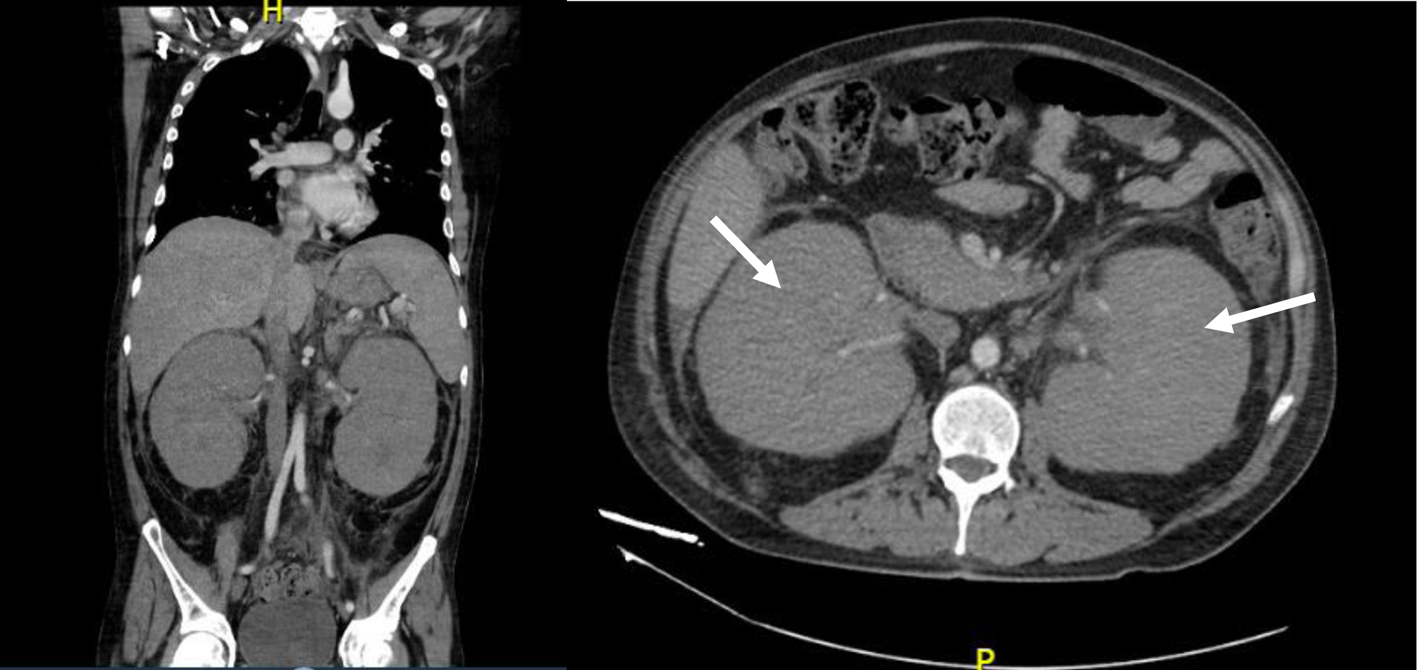 Click for large image | Figure 1. Computed tomography scan showing nephromegaly (white arrows) before kidney biopsy. |
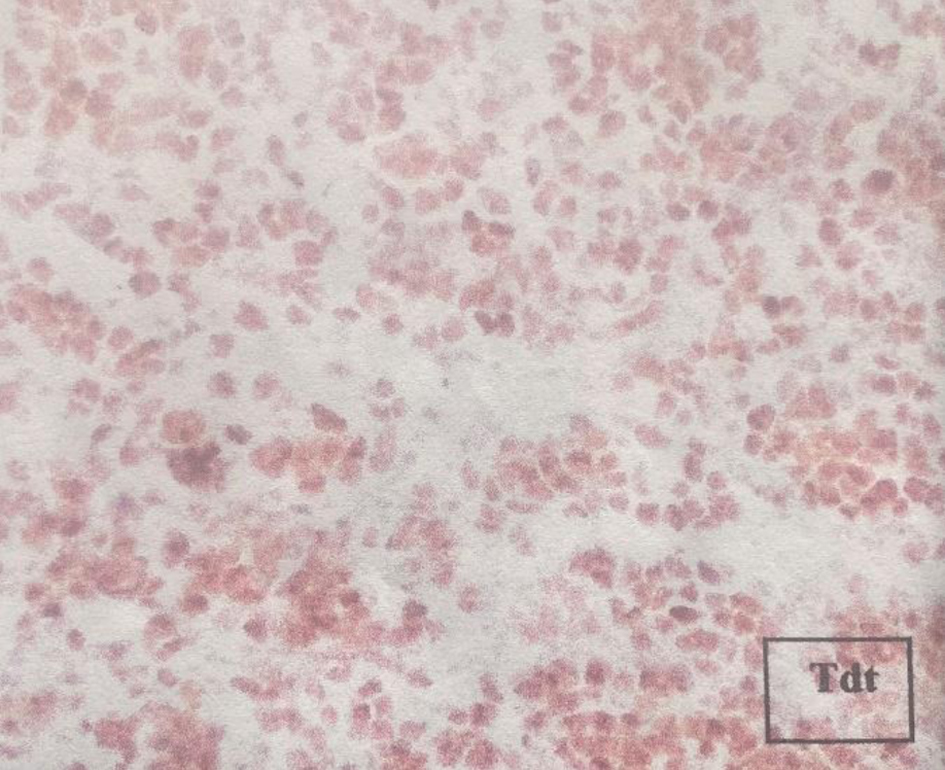 Click for large image | Figure 2. Immunohistochemical staining reporting TdT positivity in the kidney biopsy. |
Treatment
During hospitalization, high-dose diuretic treatment was started without success. The patient had worsening renal function, so he was treated with sequential hemodialysis, with clinical improvement but was dependent on renal replacement therapy. He was discharged with acceptable urea levels awaiting the result of the kidney biopsy.
When discussing with the patient the treatment options for renal relapse, the patient did not accept high-dose chemotherapy, immunotherapy and second HSCT, and he preferred palliative treatment, therefore he began low-dose chemotherapy. He was treated with 1 mg per kg prednisone for 28 days, twice a week oral methotrexate, and daily mercaptopurine; edema decreased considerably but there was not any improvement in kidney function. One week after the iEMR diagnosis, the patient began with mild respiratory symptoms, cough, sneezing, pharyngodynia, and normal pulse oximetry; he was tested for coronavirus disease 2019 (COVID-19), resulting positive. Due to the above, he was classified as mild COVID-19 and received symptomatic treatment at home, continuing with established chemotherapy.
Follow-up and outcomes
Six weeks after the iEMR, and while the patient was receiving oral chemotherapy, he presented with sudden pallor, dyspnea, and generalized purpura at the emergency department. Laboratory showed severe anemia, leukopenia and thrombocytopenia, and peripheral blood smear did not report immature cells. Bone marrow smear and biopsy demonstrated 60% of lymphoblasts. After the B-cell ALL medullary relapse was diagnosed. He died 8 weeks later from pulmonary hemorrhage due to severe thrombocytopenia. The critical events in the evolution of this patient from the diagnosis of ALL until his death are described in the timeline (Table 1), achieving an overall survival of 18 months.
 Click to view | Table 1. Timeline From ALL Diagnosis to Patient Death |
| Discussion | ▴Top |
Extramedullary involvement in patients with ALL is not an uncommon finding in those newly diagnosed [8]. The molecular mechanisms behind extramedullary relapse are still poorly understood. Leukemic cells spread to different organs via the systemic circulation, potentially infiltrating any site [13]. According to a study by Ge et al, it seems that patients with ALL have a higher incidence of EMR than those with AML after allo-SCT (12.9% versus 4.6%, respectively). In this study, the most common site of iEMR was the central nervous system in ALL. There were no cases of renal iEMR. The risk factors identified for EMR in patients with previous allo-HSCT included: extramedullary leukemia before HSCT (risk ratio (RR): 3.01, P = 0.38), hyperleukocytosis at diagnosis (RR: 3.382, P = 0.004), and peripheral blood stem cells (PBSCs) as graft source (RR: 5.4, P = 0.002). Conditioning regimen and presence of acute graft-versus-host disease (aGVHD) or chronic graft-versus-host disease (cGVHD) were not identified as risk factors [14]. Of these risk factors, our patient presented PBSC as a graft source.
Renal involvement during diagnosis or treatment of ALL is joint. Different mechanisms may explain it. These include postrenal, prerenal, or intrarenal causes of acute kidney injury or acute tubular necrosis. It seems that acute kidney injury in leukemias is common. Nonetheless, only 1% are due to infiltration from acute leukemia cells [15]. De et al reported a case of a 16-year-old male who presented with renal relapse after 36 months of completing therapy. He initially developed painless hematuria. Imagining studies revealed an exophytic mass on the left kidney [11]. Rose et al reported a case of a 37-year-old Hispanic male diagnosed with ALL and treated with chimeric antigen receptor (CAR) T-cell therapy followed by allo-HSCT. He was admitted to the hospital because of hematuria and left flank pain. The CT scan noted a mass in the kidney. Both renal biopsy and bone marrow aspirate showed infiltration by leukemic cells [12]. In our case, the patient was also a fourth-decade-old Hispanic man who had received allo-HSCT 10 months prior. It seems the reason for admission is virtually the same in all three cases. Our patient presented with proteinuria, edema, and nephromegaly, suggesting renal involvement.
Treatment for patients with isolated renal infiltration post-allo-SCT is still not well standardized. It is universally agreed that they must be treated as any iEMR. In a study by Shem-Tov et al, 41.3% of patients who had an iEMR after allo-HSCT and received dose-intensive chemotherapy achieved CR [9]. Yoon et al presented two cases of ALL with iEMR after allo-HSCT treated with blinatumomab as a bridge before receiving a second allo-HSCT. Both responded favorably to blinatumomab, with one of the cases receiving it twice [16]. It seems that blinatumomab might be the future in treating iEMR before a second allo-HSCT, but more studies are needed.
This case shows the clinical picture, the imaging studies, and the diagnosis of an ALL after an isolated relapse to the kidney. The treatment of these patients must be established immediately since the evolution to progression is fast. The choice of treatment in an eligible patient who wants intense chemotherapy could include blinatumomab as bridging therapy in an attempt to achieve a second allo-HSCT.
Learning points
ALL isolated renal relapse is an infrequent presentation, and the data are scarce. The clinical features include flank pain, hematuria, and edema; the mandatory workup includes a kidney CT scan and biopsy with immunohistochemical analysis. Because treatment is not well-standardized, renal iEMR in B-cell ALL is a complication with a lousy prognosis and usually is the herald of a bone marrow relapse. More studies are needed to standardize the epidemiology, diagnosis, and treatment.
Acknowledgments
We would like to acknowledge nephrology and pathology departments of our hospital for their collaboration, and all the residents of hematology of our center who were involved in the treatment of this patient.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was waved because the manuscript was made according to the current standard, and the patient identification was kept secret for protection purposes.
Author Contributions
Jimenez-Ochoa and Lozano-Jaramillo conceived of the presented idea. Gonzalez-Bautista and Jimenez-Ochoa developed the theory and performed the analysis. Lopez-Macias and Contreras-Serratos supervised the findings of this work and checked the English translation. All authors discussed the results and contributed to the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; allo-HSCT: allogeneic hematopoietic stem cell transplantation; CR: complete remission; GVHD: graft-versus-host disease; iEMR: isolated extramedullary relapse; LDH: lactate dehydrogenase; NSAIDs: non-steroidal anti-inflammatory drugs; PBSCs: peripheral blood stem cells; Ph-neg: Philadelphia chromosome-negative
| References | ▴Top |
- Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol. 2016;96(5):447-460.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
doi pubmed - Rafei H, Kantarjian HM, Jabbour EJ. Recent advances in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60(11):2606-2621.
doi pubmed - Jabbour E, Pui CH, Kantarjian H. Progress and Innovations in the Management of Adult Acute Lymphoblastic Leukemia. JAMA Oncol. 2018;4(10):1413-1420.
doi pubmed - Saadeh SS, Litzow MR. Hematopoietic stem cell transplant in adults with acute lymphoblastic leukemia: the present state. Expert Rev Hematol. 2018;11(3):195-207.
doi pubmed - Potdar R, Varadi G, Fein J, Labopin M, Nagler A, Shouval R. Prognostic scoring systems in allogeneic hematopoietic stem cell transplantation: where do we stand? Biol Blood Marrow Transplant. 2017;23(11):1839-1846.
doi pubmed - Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biol Blood Marrow Transplant. 2007;13(1):116-123.
doi pubmed - Geethakumari PR, Hoffmann MS, Pemmaraju N, Hu S, Jorgensen JL, O'Brien S, Daver N. Extramedullary B lymphoblastic leukemia/lymphoma (B-ALL/B-LBL): a diagnostic challenge. Clin Lymphoma Myeloma Leuk. 2014;14(4):e115-118.
doi pubmed - Shem-Tov N, Saraceni F, Danylesko I, Shouval R, Yerushalmi R, Nagler A, Shimoni A. Isolated extramedullary relapse of acute leukemia after allogeneic stem cell transplantation: different kinetics and better prognosis than systemic relapse. Biol Blood Marrow Transplant. 2017;23(7):1087-1094.
doi pubmed - Poon LM, Hamdi A, Saliba R, Rondon G, Ledesma C, Kendrick M, Qazilbash M, et al. Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(7):1059-1064.
doi pubmed - De A, Menell JS. Isolated renal relapse in acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32(2):150-151.
doi pubmed - Rose A, Slone S, Padron E. Relapsed acute lymphoblastic leukemia presenting as acute renal failure. Case Rep Nephrol. 2019;2019:7913027.
doi pubmed - Gaudichon J, Jakobczyk H, Debaize L, Cousin E, Galibert MD, Troadec MB, Gandemer V. Mechanisms of extramedullary relapse in acute lymphoblastic leukemia: Reconciling biological concepts and clinical issues. Blood Rev. 2019;36:40-56.
doi pubmed - Anthony S, Stephen FJ. Allogeneic and autologous hematopoietic stem cell transplantation for acute lymphoblastic leukemia and acute myelogenous leukemia in the adult. In: Soiffer R.J. (eds). Hematopoietic Stem Cell Transplantation. 2008:57-82.
doi - Luciano RL, Brewster UC. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis. 2014;21(1):27-35.
doi pubmed - Yoon SY, Yoon JH, Min GJ, Park SS, Park S, Lee SE, Cho BS, et al. Experience of blinatumomab salvage for patients with acute lymphoblastic leukemia presenting with isolated extramedullary relapse after previous allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55(7):1469-1472.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


