| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 5, May 2022, pages 244-248
Drug-Induced Liver Injury Secondary to Enobosarm: A Selective Androgen Receptor Modulator
Daniel Weinblatta, c, Satyajeet Royb
aDepartment of Medicine, Cooper University Health Care, Camden, NJ, USA
bDepartment of Medicine, Cooper Medical School of Rowan University - Cooper University Health Care, Camden, NJ, USA
cCorresponding Author: Daniel Weinblatt, Department of Medicine, Cooper University Health Care, Camden, NJ 08103, USA
Manuscript submitted March 24, 2022, accepted May 5, 2022, published online May 7, 2022
Short title: DILI due to Enobosarm
doi: https://doi.org/10.14740/jmc3937
| Abstract | ▴Top |
Selective androgen receptor modulators (SARMs) are compounds that bind to androgen receptors and have similar anabolic properties to anabolic steroids. Unlike anabolic steroids, which bind to androgen receptors in many tissues all over the body, individual SARMs selectively bind androgen receptors in certain tissues, but not in others. This selectivity has attracted researchers due to the possibility of using SARMs for the potential benefits of androgen receptor stimulation, such as increased muscle mass and increased bone density, while minimizing the adverse effects, such as erythrocytosis and hepatotoxicity. Enobosarm, a SARM, has been studied for use in treatment of cachexia, osteoporosis, breast and prostate cancers, and stress urinary incontinence. Enobosarm can be found in some over-the-counter muscle-building supplements. We report a 31-year-old man with no significant personal or family medical history who presented with itching and dark-colored urine for 1 week. Three weeks prior to presentation, he had begun using a muscle-building supplement containing enobosarm. Diagnostic workup concluded a drug-induced hepatocellular liver injury secondary to enobosarm, which subsequently improved after discontinuation of enobosarm-containing muscle-building supplement use. As enobosarm and other SARMs are increasingly found in the over-the-counter supplements and being studied for other clinical applications, it is important to recognize their potential for liver toxicity.
Keywords: Enobosarm; Selective androgen receptor modulators; Drug-induced liver injury; Muscle-building supplements
| Introduction | ▴Top |
Drug-induced liver injury (DILI) is a condition that is fairly common, potentially severe, but occasionally difficult to diagnose, as it is a diagnosis of exclusion. By some estimates, DILI may account for 10-50% of all cases of acute hepatitis and nearly 25% of cases of fulminant hepatic failure [1]. When acetaminophen overdose is excluded, DILI is reported to be the cause of 7-15% cases of acute liver failure in the United States and Europe [2]. DILI can be classified as intrinsic or idiosyncratic. In intrinsic DILI, drug hepatotoxicity is predictable, dose-related, and occurs within hours to days after ingestion. In idiosyncratic DILI, the injury is unpredictable, rare, and can occur even weeks after exposure. While idiosyncratic DILI may occur infrequently for a given drug, it is still a significant concern, particularly in drug development. Hepatotoxicity was the basis for 32% of drug withdrawals from the USA between 1975 and 2007 [3]. The diagnosis of DILI requires a clear history of drug ingestion and exclusion of other potential causes of liver injury. There has long been a link between the use of androgenic anabolic steroids and liver injury [4]. However, there are only a few reports of DILI related to the use of selective androgen receptor modulators (SARMs), and only one reported secondary to enobosarm, a SARM. We present a case of a patient who developed DILI after using an over-the-counter muscle-building supplement that contained enobosarm. In this case presentation, we aim to present the limited but growing evidence of the liver toxicity of SARMs that can be found in many over-the-counter muscle-building supplements.
| Case Report | ▴Top |
Investigations
A 31-year-old man presented to our primary care office with itching and dark-colored urine for 1 week. He had no personal or family history of liver disease. He reported consuming one alcoholic drink, either one beer or 2 oz of liquor, on average per week. He reported no sick contacts, no current or previous illicit drug use, no unprotected sexual intercourse, and no recent travel. The patient denied abdominal pain, nausea, vomiting, diarrhea, fevers or chills. He had never experienced similar symptoms before. The patient reported that 3 weeks prior to the presentation, he had started taking an over-the-counter muscle-building supplement that contained the main ingredient enobosarm, a SARM. There were no other ingredients in the supplement. After taking the supplement for 2 weeks, he developed itching throughout his body and dark-colored urine. He did not have a rash or yellowing discoloration of his skin or eyes (Fig. 1).
 Click for large image | Figure 1. Timeline of presenting illness. |
On physical exam, the patient appeared healthy without distress. His vital signs were normal with blood pressure of 118/76 mm Hg, pulse of 63 beats per minute, respiratory rate of 16 breaths per minute, oral temperature of 98 °F, and room air SpO2 of 98%. His body mass index (BMI) was 33.86 kg/m2. The abdominal exam revealed no hepatomegaly or splenomegaly, no tenderness, and no distention. There was no jaundice or scleral icterus. The remainder of the physical exam was unremarkable.
Diagnostic tests at the patient’s initial presentation were notable for elevated serum alanine aminotransferase (ALT) of 346 U/L, elevated aspartate aminotransferase (AST) of 110 U/L, alkaline phosphatase of 123 U/L, and total bilirubin of 0.5 mg/dL. The serum albumin was 4.9 g/dL and international normalized ratio (INR) was 1.0, both within normal limits. The rest of his comprehensive metabolic panel, and complete blood count showed no abnormality.
Diagnosis
The differential diagnosis of our patient’s initial presentation included DILI, non-alcoholic fatty liver disease, autoimmune hepatitis, primary biliary cirrhosis, hereditary hemochromatosis, primary sclerosing cholangitis, Wilson’s disease, biliary obstruction secondary to malignancy, choledocholithiasis, Mirizzi syndrome, Budd-Chiari syndrome, and acute hepatitis A virus infection.
Hepatitis A and C virus antibody tests were negative. Hepatitis B virus surface antibody was positive while hepatitis B surface antigen, core antibody and e-antigen were negative, consistent with prior vaccination. His baseline liver tests conducted 3 years prior as part of routine blood tests were all within normal limits. Toxicology screening tests were negative. A right upper quadrant abdominal ultrasound with venous Doppler demonstrated an enlarged fatty liver, with a length of 199 mm at the mid-clavicular line. There was normal venous flow and there were no discrete lesions, gallstones, or bile duct dilation.
The other results were notable for normal serum iron level, serum ferritin level of 447 ng/mL, negative anti-smooth muscle antibody, negative anti-mitochondrial antibody, normal ceruloplasmin level, negative anti-nuclear antibody, negative human homeostatic iron regulator (HFE) gene testing, and normal gamma-glutamyl transferase.
We conducted a detailed analysis of the differential diagnosis. Non-alcoholic fatty liver disease was a possibility in this patient given a BMI of 33 kg/m2, though it is typically asymptomatic and would not cause this degree of elevation in liver enzymes. Other, less common conditions such as autoimmune hepatitis, primary biliary cirrhosis, hereditary hemochromatosis, and Wilson’s disease were excluded on the basis of negative laboratory testing. While the ferritin was slightly elevated at 447 ng/mL, the transferrin saturation was 21%. In men with hereditary hemochromatosis, the transferrin saturation is typically greater than 45%. This suggests that ferritin was elevated as an acute phase reactant rather than due to iron overload. This was also supported by a negative HFE gene testing. Primary biliary cirrhosis was less likely due to the alkaline phosphatase level being less than 1.5 times the upper limit of normal. Primary sclerosing cholangitis was also thought to be less likely due to the hepatocellular pattern of liver injury, with an R factor of 7.5. Choledocholithiasis and Mirizzi syndrome could cause elevations in aminotransferases, but would typically present with a right upper quadrant pain in addition to symptoms and laboratory findings of cholestasis. Moreover, these diagnoses were ruled out due to negative ultrasonographic findings. Biliary obstruction secondary to malignancy was thought to be less likely in an otherwise healthy young man and there was more of a hepatocellular injury pattern rather than cholestasis. Acute biliary obstruction was also ruled out based on the ultrasonographic findings. Budd-Chiari syndrome was also less likely given the lack of abdominal pain in the patient’s presentation, and a normal flow through the hepatic vein on the ultrasonography. An acute viral hepatitis was also ruled out based on the negative serological test results. We finalized the diagnosis as DILI secondary to enobosarm ingestion, given the appearance of the patient’s symptoms shortly after ingestion of the over-the-counter muscle-building supplement which contained enobosarm as the main ingredient.
Treatment
The muscle-building supplement containing enobosarm was discontinued. A short course of cyproheptadine was prescribed to alleviate itching which offered symptomatic improvement. The patient was advised to avoid consumption of alcohol-containing beverages and over-the-counter supplements.
Follow-up and outcomes
The patient was followed up in the office weekly for 2 weeks followed by monthly for 3 months, and subsequently at 6 - 12 months. Both the itching and dark-colored urine resolved in the weeks following the patient’s discontinuation of enobosarm supplement use. A repeat laboratory testing was obtained 3 days after the initial presentation. Aminotransferases showed improvement, with ALT 184 U/L, AST 36 U/L, alkaline phosphatase 101 U/L, and total bilirubin 0.2 mg/dL. Another follow-up liver profile after 7 days showed further improvement, with ALT 90 U/L, AST 24 U/L, alkaline phosphatase 95 U/L, and total bilirubin 0.5 mg/dL. A liver biopsy was initially planned, however due to the rapid improvement of his symptoms after cessation of enobosarm and the favorable trend of the follow-up diagnostic studies, the patient and the care team decided not to pursue the liver biopsy. A follow-up liver profile 10 months later showed normalization of the ALT and AST at 25 U/L and 18 U/L, respectively (Fig. 2).
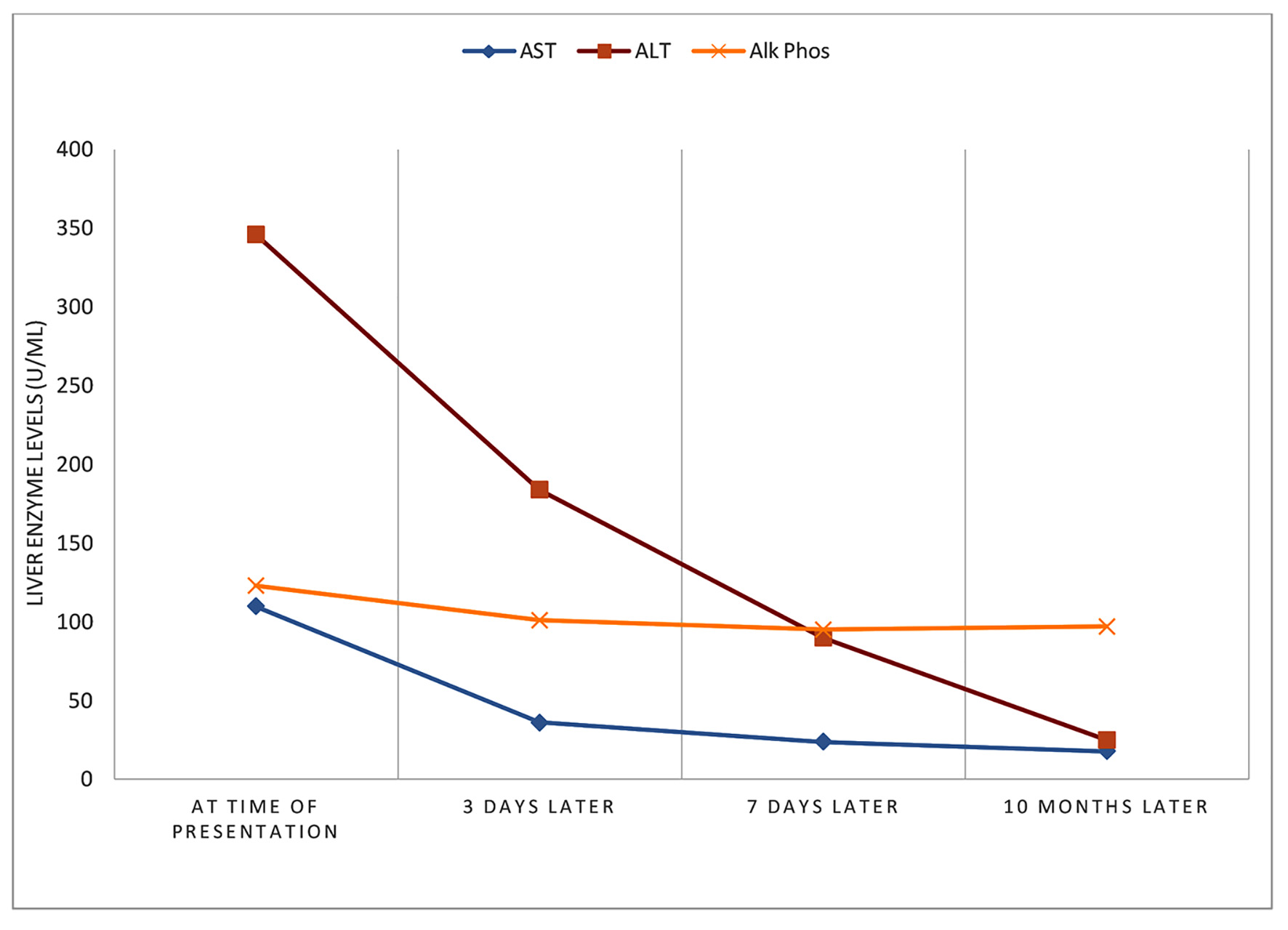 Click for large image | Figure 2. Course of liver injury. |
| Discussion | ▴Top |
Diagnosis of DILI can be challenging, as it requires exclusion of other causes of liver disease, as well as a clear history of drug exposure. There should also be improvement in liver injury following cessation of the drug, and recurrence of injury with repeat challenge with the drug, though this is generally not recommended. There are numerous drugs and chemicals either proven or implicated as causes of liver injury. Many herbal and dietary supplements contain chemical compounds that are included on that list. Androgenic anabolic steroids (AAS) are among the compounds that are known to cause a range of liver injuries, from transient transaminase elevations, to acute “bland cholestasis”, to peliosis hepatis. Long-term use of AAS has also been linked to the development of hepatic tumors [4]. SARMs, on the other hand, are compounds that bind to androgen receptors, akin to the action of selective estrogen receptor modulator (SERMs) on estrogen receptors.
Enobosarm, also known as Ostarine or MK-2866, was first discovered in the 1990s, and has been studied for use in treatment of cachexia, osteoporosis, breast and prostate cancers, and stress urinary incontinence [5, 6]. In animal studies, enobosarm increased muscle mass and bone density while having limited effects on other androgen-responsive tissues including the prostate and seminal vesicles. The animal studies also found that the prostate size was reduced in male rats at doses that increased muscle mass. In a phase I study in 48 healthy 18- to 45-year-old males and 23 elderly males with truncal obesity, Dalton and associates administered enobosarm for 14 days and reported increased lean body mass and favorable tolerability profile without clinically apparent adverse effects [7]. Interestingly, in the phase II trial of enobosarm for treatment of cachexia, increases in ALT were noted in 20.8% of the individuals taking the highest and most effective study dose of 3 mg [7]. In 2017, the United State Food and Drug Administration (FDA) issued a warning to several supplement manufacturers for including SARMs in products marketed and labeled as dietary supplements. The warning not only noted the potential for liver toxicity, but it also suggested an increased risk of heart attack and stroke [8].
There are only a few published case reports of SARMs being implicated as the causes of liver injury. Flores et al published a case series of two patients who developed liver injury after taking SARMs. In the first of those cases, an otherwise healthy 24-year-old patient consumed the gym product Ligandrol for 9 weeks. He also had an additional history of binge-consumption of alcohol. The patient had similar elevations in ALT and AST as observed in our patient, along with elevation of total bilirubin [9]. However, our patient’s bilirubin levels were in the normal range, and he consumed alcohol socially and occasionally. In the second case, a 49-year-old man consumed RAD-140 for 4 weeks, 4 months prior to presentation. He was also on venlafaxine for 11 months. He had a mixed hepatocellular-cholestatic liver injury, and was treated with ursodiol and cholestyramine after cessation of venlafaxine and RAD-140. Both patients’ liver enzymes normalized in the subsequent months [9]. Lastly, there is one recently published report of a patient developing liver injury following enobosarm use. In that report, the patient had been using a supplement containing enobosarm for 2 months. Interestingly, analysis of the liver tests revealed an R factor of 0.8, indicating a predominant cholestatic liver injury. He also underwent a liver biopsy, with histopathologic findings of hepatic ductular reaction with minimal inflammation and significant cholestasis. The patient in that case had a similar improvement in liver injury over several months after he stopped taking the supplement [10]. The discrepancy between the types of liver injury observed in that case and the one presented here may be due to the duration or quantity of enobosarm use.
A liver biopsy in the case of our patient could have helped to define the mechanism of liver injury caused with enobosarm either by demonstrating a similar histopathology, or by revealing an alternative pattern of damage. However, a biopsy would have been invasive and would not have been likely to change the diagnosis or management in this case, given the improvements in the patient’s liver enzymes. For these reasons as well as the patient’s preference, a liver biopsy was not pursued. Additionally, our patient’s causality assessment scores by Roussel Uclaf Causality Assessment Method was 7, which falls into the category of “probable” (score 6 - 8) [11]. When comparing RUCAM to other causality assessment instruments, the other terminology that corresponds with “RUCAM probable” is documented as “highly probable” [11]. Further analysis of the study by Dalton and associates, in which they recruited 120 healthy elderly men (aged greater than 60 years) and postmenopausal women and conducted a 12-week double-blind, placebo-controlled phase II clinical trial to evaluate the role of enobosarm in lean body mass and physical function, revealed noteworthy observations [7]. Although the authors concluded a dose-dependent improvement in total lean body mass and physical function, and found similar adverse effects between the intervention group and the placebo group, nevertheless one subject in the intervention group discontinued the trial due to rise in a four times upper limit of normal rise in the ALT. Additionally, there were seven other subjects in the intervention group who demonstrated smaller rise in the ALT, as well. The effects of 1.0 or 3.0 mg of enobosarm on lean body mass have also been studied in another randomized, controlled phase II trial; however, the study did not report any particular liver-related adverse events [12].
Although the exact mechanism of liver injury due to enobosarm is unclear, several possible mechanisms have been suggested based on the observations made in the DILI associated with other SARMs. Idiosyncrasy happens to play a major role in the majority of the cases of DILI due to other SARMs. The rarity of reported cases of DILI secondary to enobosarm, a predominantly lymphocytic infiltrate in the liver tissue in the few reported cases of other SARMs, and lack of association between the length of use and the severity of the liver injury make the idiosyncratic immune response as the most plausible mechanism of liver injury. The biochemical response in the form of mixed-type hepatocellular-cholestatic injury and the reported histological findings in the few reported cases with other SARMs indicate combined targeting of hepatocytes and cholangiocytes by the immune response, which can be explained by the idiosyncratic immune response [13].
We conclude that our patient suffered a DILI caused by the muscle-building supplement containing enobosarm. The laboratory findings on presentation suggested a hepatocellular pattern with predominant transaminase elevation. Our case report highlights the need for further study of the liver damage caused by SARMs in light of their increasing popularity and potential for misuse as body-building products.
Learning points
SARMs, such as enobosarm, are an emerging class of compounds that have the potential for both therapeutic and recreational use, but appear to carry a risk of liver injury.
Patients presenting with abnormal liver enzymes should be asked about their use of supplements, particularly those patients involved in weight-lifting and muscle or body building.
Further research is needed to characterize the effects of enobosarm on the liver.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no financial disclosure to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The patient’s informed consent for publication was obtained.
Author Contributions
DW and SR provided patient care. DW drafted the manuscript. SR contributed in revising the manuscript critically for improved intellectual content, and final approval for the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
SARM: selective androgen receptor modulator; DILI: drug-induced liver injury; ALT: alanine aminotransferase; AST: aspartate aminotransferase
| References | ▴Top |
- Zimmerman HJ. Drug-induced liver disease. Clin Liver Dis. 2000;4(1):73-96, vi.
doi - Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806-815.
doi pubmed - Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, Devarbhavi H, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5(1):58.
doi pubmed - LiverTox: clinical and research information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Androgenic Steroids. [Updated May 30, 2020]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548931/
- Solomon ZJ, Mirabal JR, Mazur DJ, Kohn TP, Lipshultz LI, Pastuszak AW. Selective androgen receptor modulators: current knowledge and clinical applications. Sex Med Rev. 2019;7(1):84-94.
doi pubmed - Business Wire. GTx announces top-line results from placebo-controlled ASTRID trial of enobosarm in women with stress urinary incontinence. GTx announces top-line results from placebo-controlled ASTRID Trial of Enobosarm in Women with Stress Urinary Incontinence | Business Wire. Available from: https://www.businesswire.com/news/home/20180921005082/en/. Published September 21, 2018. Accessed December 2, 2020.
- Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2(3):153-161.
doi pubmed - The United States Food and Drug Administration. FDA in brief: FDA warns against using SARMs in body-building products. 2017. Available from: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-against-using-sarms-body-building-products.
- Flores JE, Chitturi S, Walker S. Drug-induced liver injury by selective androgenic receptor modulators. Hepatol Commun. 2020;4(3):450-452.
doi pubmed - Bedi H, Hammond C, Sanders D, Yang HM, Yoshida EM. Drug-induced liver injury from enobosarm (Ostarine), a selective androgen receptor modulator. ACG Case Rep J. 2021;8(1):e00518.
doi pubmed - LiverTox: clinical and research information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Roussel Uclaf Causality Assessment Method (RUCAM) in drug induced liver injury. [Updated May 4, 2019]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548272.
- Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, Johnston MA, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14(4):335-345.
doi - Kolaric TO, Nincevic V, Smolic R, Smolic M, Wu GY. Mechanisms of hepatic cholestatic drug injury. J Clin Transl Hepatol. 2019;7(1):86-92.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


