| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 12, Number 8, August 2021, pages 315-318
Two Acute Liver Injuries in a Patient With Malnutrition
Amanda Sua, Monica Choeb, Jacqueline E. Birknessc, Berkeley Limketkaid, Po-Hung Chena, e
aDivision of Gastroenterology & Hepatology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA
bJohns Hopkins University School of Nursing, Baltimore, MD, USA
cDepartment of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
dDivision of Digestive Diseases, Department of Medicine, UCLA School of Medicine, Los Angeles, CA, USA
eCorresponding Author: Po-Hung Chen, Division of Gastroenterology & Hepatology, Department of Medicine, Johns Hopkins University School of Medicine, 1830 E. Monument Street, Suite 429, Baltimore, MD 21287, USA
Manuscript submitted May 15, 2021, accepted June 3, 2021, published online July 3, 2021
Short title: Acute Liver Injuries in Patient With Malnutrition
doi: https://doi.org/10.14740/jmc3713
| Abstract | ▴Top |
Chronic starvation and refeeding have been associated with liver injury (LI). We present a patient with anorexia nervosa who exhibited both phenomena of malnutrition-related LI. At presentation, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were elevated at 154 and 136 U/L, respectively, and rose rapidly to peaks of 750 and 638 U/L, respectively, as nutrition was introduced. Mechanisms of starvation-related LI include impaired degradation and secretion of lipids, as well as starvation-induced autophagy. LI during refeeding may be related to rapid increase in glucose availability. These phenomena are crucial to consider in patients with chronic starvation undergoing refeeding.
Keywords: Malnutrition; Refeeding; Hepatic steatosis; Liver injury
| Introduction | ▴Top |
Malnutrition is a rare, but known, cause of hepatic dysfunction. Chronic starvation can lead to a spectrum of histologic changes, ranging from glycogenic insufficiency to fatty deposition in hepatocytes. These changes are further reflected in liver biochemical derangements, previously described in patients with anorexia nervosa [1]. While nourishment ultimately leads to the resolution of these laboratory abnormalities in most cases, physiologic changes in refeeding can also cause an acute but temporary worsening of transaminases. We present a patient who exhibited both phenomena of nutrition-related transaminase abnormalities.
| Case Report | ▴Top |
Investigations
A 45-year-old woman presented to our hospital with a failure to thrive. She was in her usual state of health until 6 years prior to admission. She developed a poor appetite and severe food aversion, and her weight declined from 54.5 kg to her admission weight of 34.2 kg. The patient reported chronic constipation and intolerance of many foods, including oil, fats and meats. She denied dysphagia, odynophagia, abdominal bloating, pain, or diarrhea. The personal and family medical histories were unremarkable. She was not on any prior medications or supplements. She denied tobacco, alcohol, or illicit drug use.
Diagnosis
On physical examination, the patient was afebrile with normal vital signs. She measured 5 feet 1 inch tall and weighed 34.2 kg, with a body mass index (BMI) of 14.2 kg/m2. There was profound cachexia. There was no hepatosplenomegaly, abdominal tenderness, or distention. A comprehensive gastrointestinal workup including computed tomography of the abdomen and pelvis, videofluoroscopic swallowing study, esophagogastroduodenoscopy, colonoscopy, gastric emptying study, and small bowel series, was unremarkable. Complete blood count was normal. Notable abnormalities on the metabolic panel were the sodium at 129 mEq/L (normal 135 - 148), chloride at 88 mEq/L (normal 96 - 109), and creatinine at 0.4 mg/dL (normal 0.5 - 1.2). The aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were 154 U/L (normal 0 - 31) and 136 U/L (normal 0 - 31), respectively. The serum bicarbonate, urea nitrogen, glucose, total bilirubin, alkaline phosphatase (ALP), albumin and prealbumin were normal. Psychiatry was consulted, and she was diagnosed with anorexia nervosa.
The patient was started on a regular diet on day 1 of hospitalization, with estimated needs of 1,345 - 1,598 calories per day and 40 - 47 g of protein per day. However, given her gastrointestinal symptoms and laboratory abnormalities, several tests were pursued which required alterations in her diet during the initial week of hospitalization. For the most part, she was nil per os (NPO) status or ordered for a clear liquid diet while workup was pending until day 8 of hospitalization. Thus, refeeding was determined to have started on day 8 of hospitalization. She was initially ordered a vegan diet per her request, with subsequent liberalization to a regular diet. A calorie count performed on days 14 - 16 of hospitalization revealed she was consuming almost 100% of all food trays. Total parenteral nutrition was discussed early during her hospitalization, given food intolerance and disruptions in oral nutrition; however, it was not pursued. Notably, her weight initially decreased from her admission weight of 34.2 kg to a nadir around 30.8 - 31.0 kg on days 10 - 13 of hospitalization. Her weight began to increase starting day 14 of hospitalization or day 7 of refeeding.
On hospitalization day 6 (i.e., before refeeding), the patient’s AST and ALT rose steeply to 671 and 362 U/L, respectively. They peaked at 750 and 638 U/L, respectively, on hospitalization day 10, 2 days after refeeding was initiated (Fig. 1). Additional laboratory workup for the etiology of liver injury (LI) included hepatitis A, B, and C serologies, which were negative. Serum ceruloplasmin and copper were normal. Ferritin and serum iron were mildly elevated. Anti-mitochondrial antibody titers were slightly elevated at 1:20 dilution, but this was considered a false positive with negative anti-nuclear and anti-smooth muscle antibodies. The immunoglobulin profile was also normal. Acetaminophen levels were undetectable. A right upper quadrant ultrasound was normal. A percutaneous liver biopsy was performed on hospitalization day 11, 3 days after refeeding; histology revealed mild glycogen depletion, marked ceroid pigment around the central veins, and scattered hepatocellular atrophy (Fig. 2). There was no histologic evidence of hepatic steatosis, inflammation, or significant fibrosis.
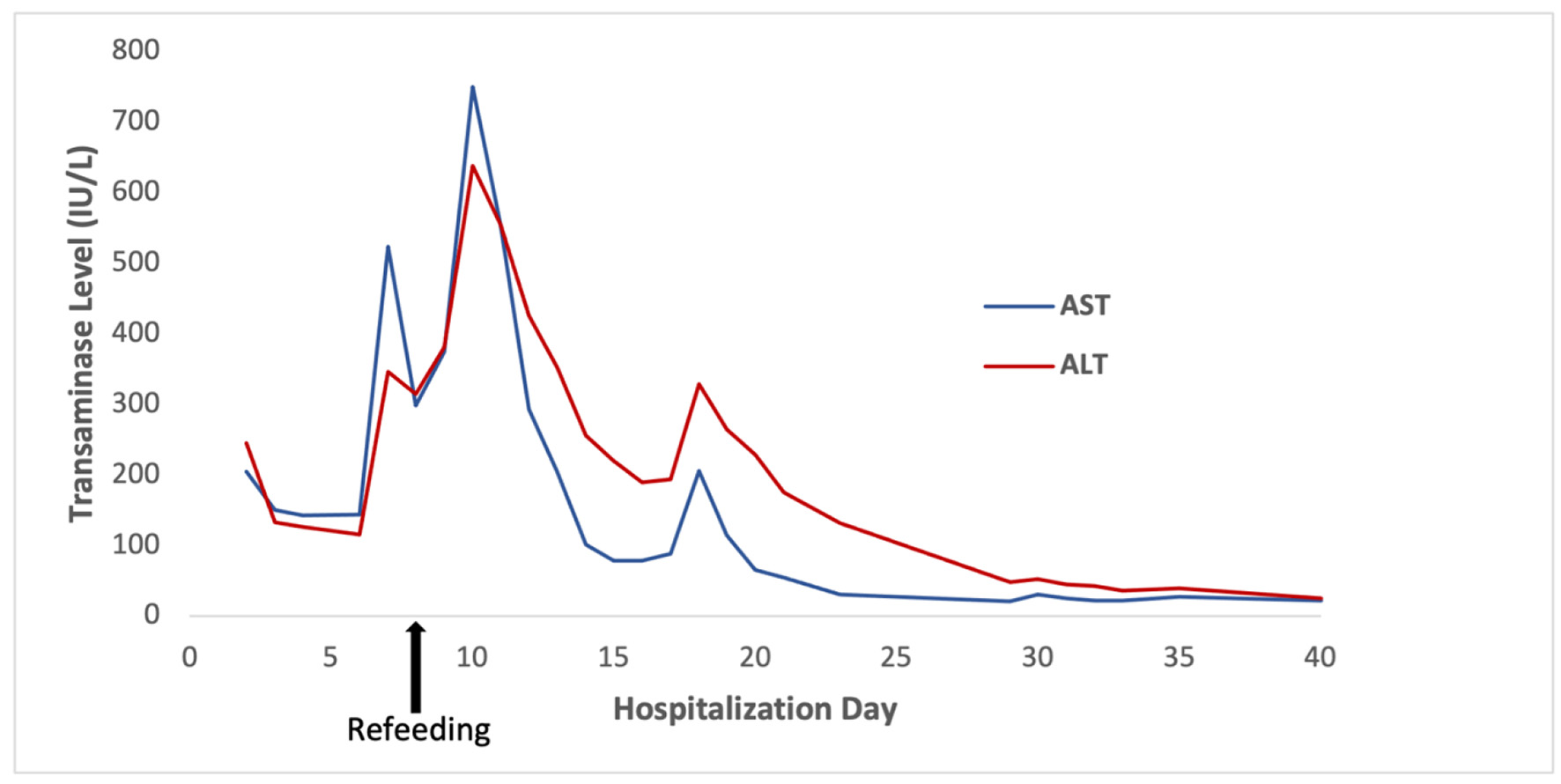 Click for large image | Figure 1. Transaminase levels throughout the patient’s hospitalization, with peak levels of AST 750 U/L and ALT 638 U/L on hospitalization day 10, 2 days after refeeding was initiated. ALT: alanine aminotransferase; AST: aspartate aminotransferase. |
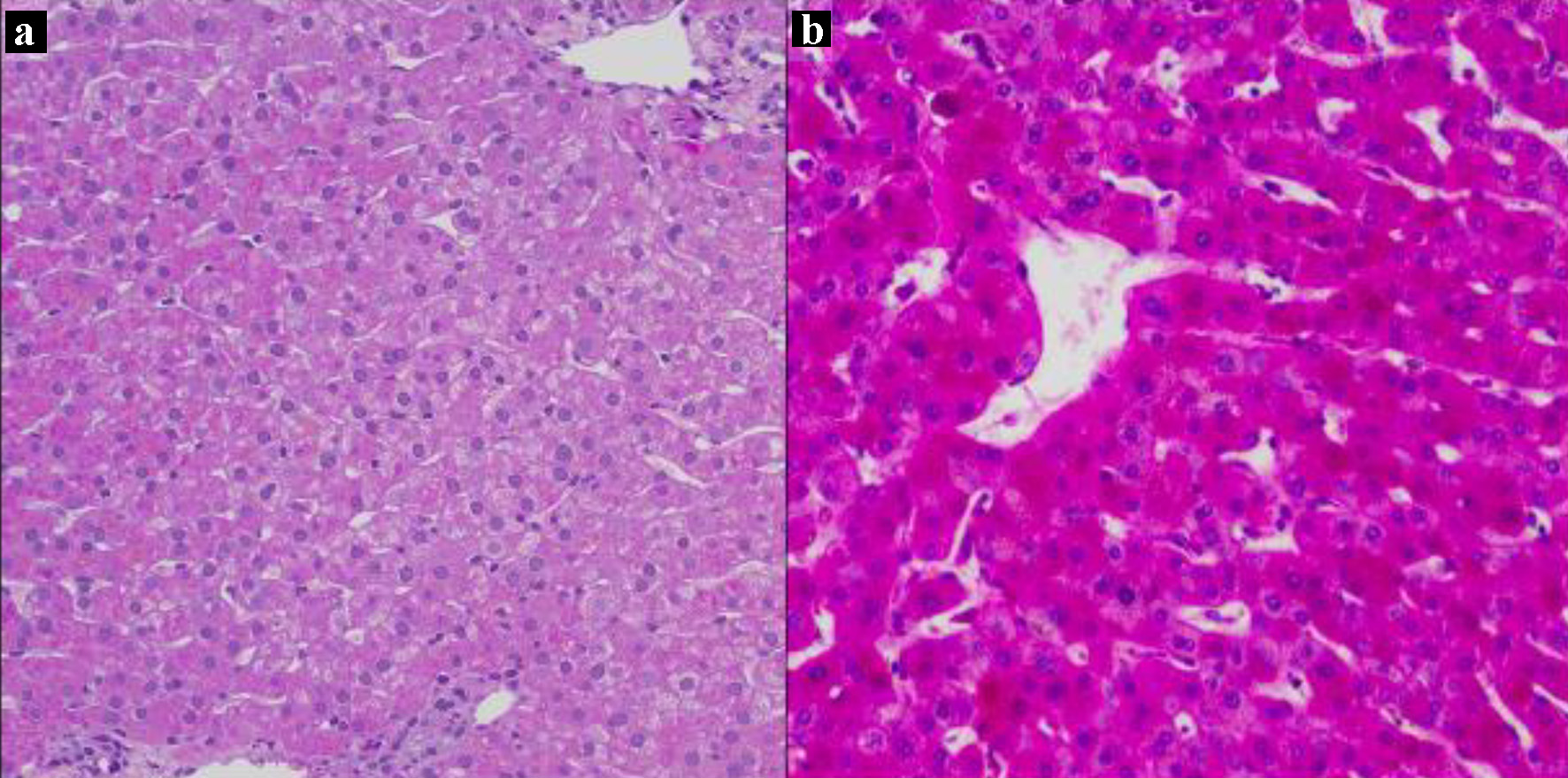 Click for large image | Figure 2. Liver biopsy on day 11 of hospitalization. Periotic acid-Schiff (PAS) stains without diastase, × 200. (a) Liver biopsy from the patient showing faint staining with PAS consistent with glycogen depletion, and an abundant amount of ceroid material pigments around the central veins. (b) Liver biopsy from a patient with normal glycogen stores for comparison. |
Follow-up and outcomes
The patient continued to tolerate a regular diet and gain weight during her hospital course. She was eventually transferred to an Eating Disorders Unit for monitoring and treatment. She then started on the nutrition protocol for eating disorders with a slow increase in daily nutrition from 1,500 calories per day to 4,000 calories per day by day 40 of hospitalization. Over the subsequent 2 months, the patient’s weight increased from 34.2 kg (BMI 14.2 kg/m2) to 38.4 kg (BMI 16.0 kg/m2), and her transaminases normalized.
| Discussion | ▴Top |
Malnutrition is known to cause hepatic dysfunction and even severe liver failure [2]. One of the first reports linking anorexia nervosa to hepatic dysfunction included two anorexic women found to have elevated AST and ALT, which resolved with increased body weights [1]. A prospective study of 101 anorexic Japanese women found that BMI negatively correlated with both AST and ALT levels prior to refeeding [3].
Elevations in serum transaminases may stem from malnutrition-related hepatic steatosis. One hypothesis has been the impaired degradation and secretion of lipids, resulting in the depletion of several amino acids [4]. In carnitine deficiency, for example, long-chain acyl-CoA cannot be shuttled into the mitochondrial matrix for β-oxidation and is instead shunted toward triglyceride and very-low-density lipoprotein synthesis [5]. A recent case report also noted the presence of abundant lipofuscin and hepatic steatosis in a patient with a history of Roux-en-Y gastric bypass who presented with severe protein-calorie malnutrition [6]. Our patient, however, did not have evidence of hepatic steatosis on liver biopsy.
Another proposed mechanism of LI is starvation-induced autophagy [2]. Autophagy involves the lysosomal degradation of cellular components into potentially reusable subunits; it has an adaptive cytoprotective role under stress conditions [7]. In nutrient deprivation, autophagy can delay cell death by recycling endogenous metabolites and even suppressing apoptosis [8]. An increase in hepatocyte permeability during autophagic cell death has been posited as a reason for elevated serum transaminases in the absence of significant cellular necrosis on histology [2].
Refeeding
Interestingly, our patient also had an acute and sharp rise in transaminase levels during her hospitalization that coincided with increased oral intake, though it preceded her increase in weight. LI during the refeeding phase is hypothesized to be related to a rapid increase in glucose availability leading to fat deposition [3, 9]. In a study of 67 patients with anorexia nervosa and normal ALT on admission, 32 (48%) developed elevated ALT during oral refeeding [9]. Similar to other studies, the degree of ALT elevation was mild compared to starvation-related ALT levels prior to refeeding. One study showed that the quantity of initially prescribed calories was associated with increased odds of elevated liver enzymes in 14 patients, further implicating increased nutrition as a causative factor [10].
Normalization
While on the Eating Disorders Unit, our patient’s serum transaminases progressively normalized, coincident with continued oral intake and an increase in BMI. The improvement presumably stemmed from the resolution of chronic and acute insults: malnutrition and acute refeeding.
In summary, severe malnutrition and acute refeeding are distinct entities of LI relevant to the malnourished patient. In this case, the peak in AST and ALT occurred shortly after the start of refeeding but before the nadir in the patient’s weight, so it is unclear whether it was malnutrition or refeeding, or both, that led to the acute rise. Both are important considerations on the differential diagnosis when evaluating a malnourished patient with abnormal transaminases. Early risk stratification and close monitoring may help minimize acute hepatic injury and other serious adverse events in patients with eating disorders and malnutrition.
Learning points
Both chronic starvation and refeeding have been associated with LI. In chronic starvation, malnutrition may lead to hepatic steatosis due to impaired degradation and secretion of lipids. Starvation-induced autophagy is another potential mechanism of LI, with increased hepatocyte permeability during this phase potentially causing elevations in AST and ALT. Reintroducing nutrition may also result in rises in AST and ALT, possibly due to a rapid increase in glucose availability, though the mechanism is less clear. These liver injuries are crucial for clinicians to recognize in patients with malnutrition or those undergoing refeeding. Fortunately, they often resolve with conservative management.
Acknowledgments
Dr. Robert A. Anders provided assistance with the histologic exam.
Financial Disclosure
We gratefully acknowledge funding by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number K23AA028297 (PC) and Gilead Sciences Research Scholars Program in Liver Disease - The Americas (PC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The patient was not able to be contacted for consent despite multiple attempts to contact her and her family by phone numbers listed in the medical chart.
Author Contributions
PC and BL: study concept and design; PC and AS: acquisition of data; AS, MC, PC, BL, and JB: analysis and interpretation of data; AS, PC, BL, MC, and JB: critical revision of the manuscript for important intellectual content; PC, BL, and JB: administrative, technical, or material support, study supervision.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BMI: body mass index; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase
| References | ▴Top |
- Nordgren L, von Scheele C. Hepatic and pancreatic dysfunction in anorexia nervosa: a report of two cases. Biol Psychiatry. 1977;12(5):681-686.
- Rautou PE, Cazals-Hatem D, Moreau R, Francoz C, Feldmann G, Lebrec D, Ogier-Denis E, et al. Acute liver cell damage in patients with anorexia nervosa: a possible role of starvation-induced hepatocyte autophagy. Gastroenterology. 2008;135(3):840-848, 848 e841-843.
doi - Ozawa Y, Shimizu T, Shishiba Y. Elevation of serum aminotransferase as a sign of multiorgan-disorders in severely emaciated anorexia nervosa. Intern Med. 1998;37(1):32-39.
doi - Di Giovanni V, Bourdon C, Wang DX, Seshadri S, Senga E, Versloot CJ, Voskuijl W, et al. Metabolomic changes in serum of children with different clinical diagnoses of malnutrition. J Nutr. 2016;146(12):2436-2444.
doi - Spaniol M, Kaufmann P, Beier K, Wuthrich J, Torok M, Scharnagl H, Marz W, et al. Mechanisms of liver steatosis in rats with systemic carnitine deficiency due to treatment with trimethylhydraziniumpropionate. J Lipid Res. 2003;44(1):144-153.
doi - Kim GN, Ho S, Saulino D, Liu X. Severe protein-calorie malnutrition-associated hepatic steatosis in a woman who had Roux-en-Y Gastric bypass for morbid obesity thirteen years ago. Gastroenterology Res. 2021;14(2):129-137.
doi - Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3-12.
doi - Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25(3):1025-1040.
doi - Imaeda M, Tanaka S, Fujishiro H, Kato S, Ishigami M, Kawano N, Katayama H, et al. Risk factors for elevated liver enzymes during refeeding of severely malnourished patients with eating disorders: a retrospective cohort study. J Eat Disord. 2016;4:37.
doi - Nagata JM, Park KT, Colditz K, Golden NH. Associations of elevated liver enzymes among hospitalized adolescents with anorexia nervosa. J Pediatr. 2015;166(2):439-443 e431.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


