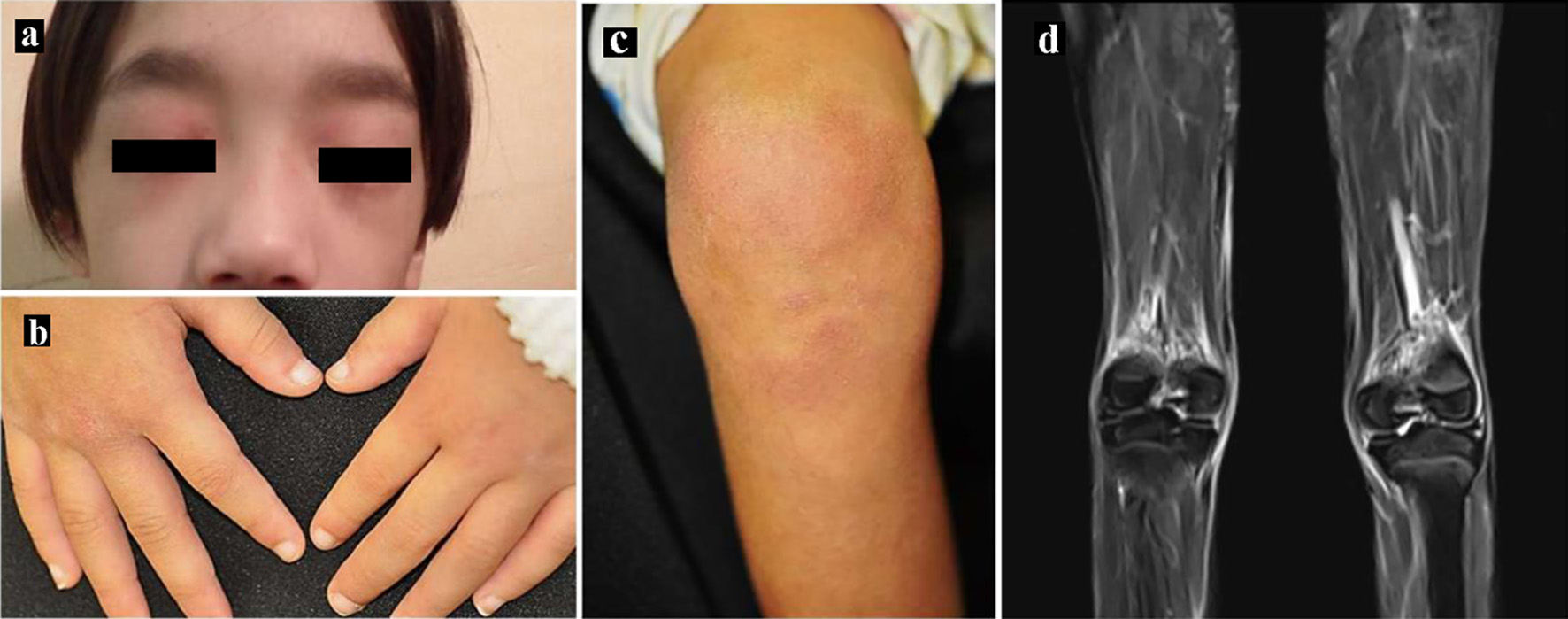
Figure 1. Skin manifestations. (a) Heliotrope rash, (b) Gottron’s papules and (c) erythema over the knees. (d) Magnetic resonance imaging showing T2-weighted and short tau inversion recovery (STIR) images of the lower limbs.
| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 6, June 2022, pages 290-296
A Continuous Increase in CXC-Motif Chemokine Ligand 10 in a Case of Anti-Nuclear Matrix Protein-2-Positive Juvenile Dermatomyositis
Figures

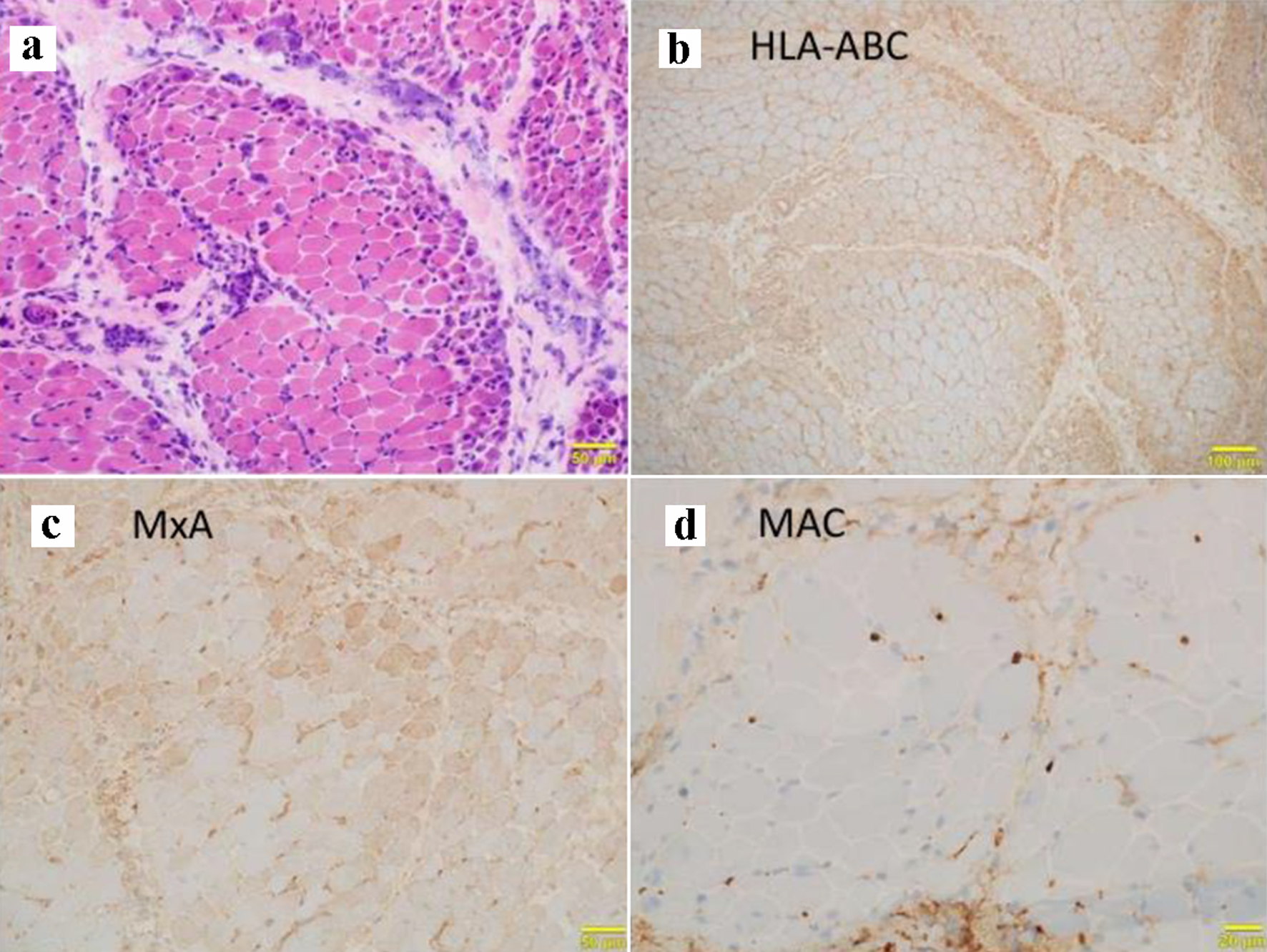
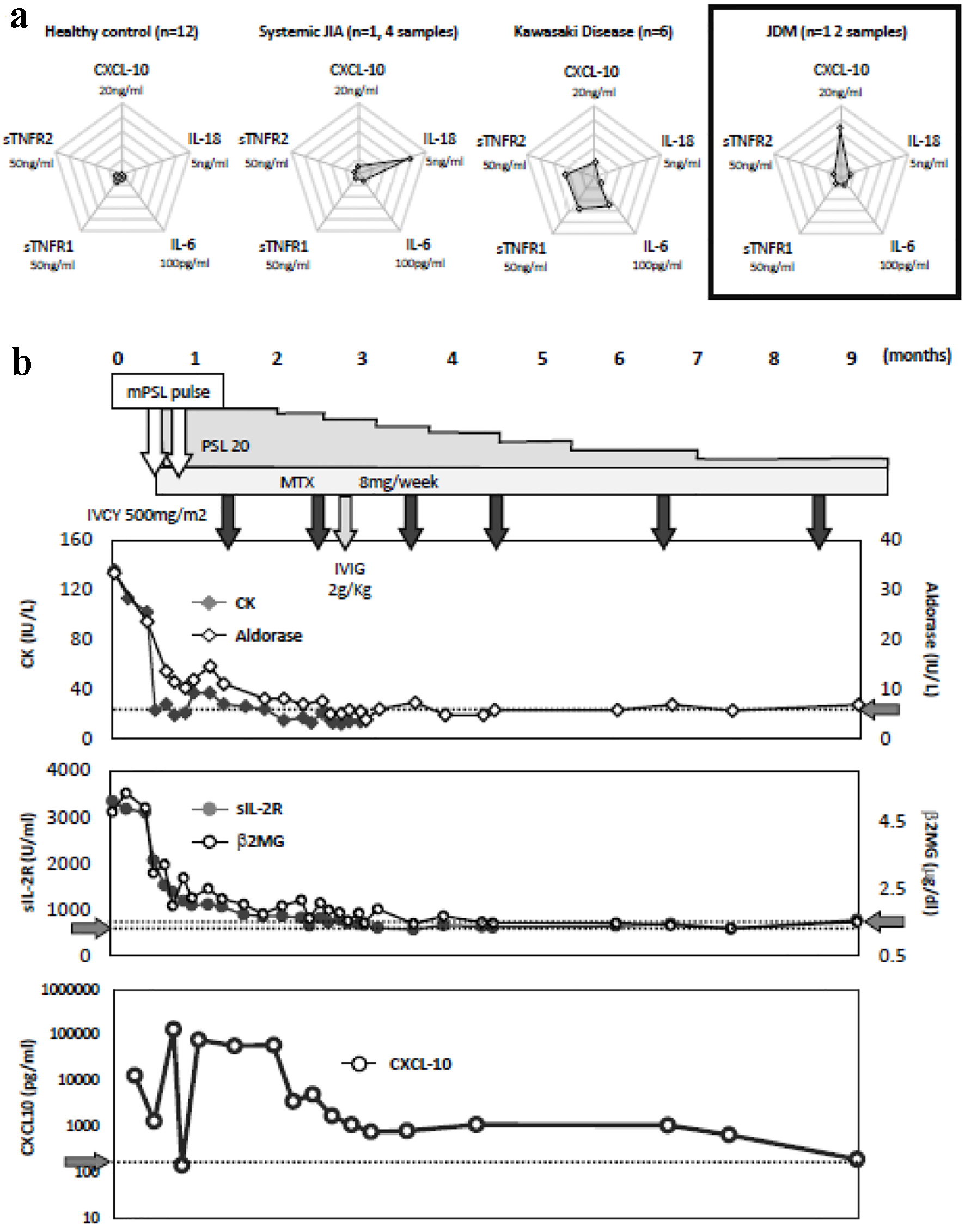
Table
| WBC: white blood cell count; RBC: red blood cell count; Hb: hemoglobin; PLT: platelets; PT-INR: prothrombin time-international normalized ratio; APTT: activated partial thromboplastin time; Fib: fibrinogen; AT III: antithrombin III; ds: double-stranded; Sm: Smith; SS: Sjogren’s syndrome; CCP: cyclic citrullinated peptide; RNP: ribonucleoprotein; TP: total protein; AST: aspartate aminotransferase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; CK: creatine kinase; BUN: blood urea nitrogen; NXP: nuclear matrix protein; ARS: aminoacyl-tRNA synthetases; MDA5: melanoma differentiation associated gene-5; Ig: immunoglobulin; sIL-2R: soluble interleukin-2 receptor; TIF1γ: transcriptional intermediary factor 1γ; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; β2MG: β2-microglobulin; NTproBNP: N-terminal proB-type natriuretic peptide. | |||||
| WBC | 5,450/µL | TP | 8.4 g/dL | CRP | 0.38 mg/dL |
| Neutrophils | 3,270/µL | Albumin | 3.3 g/dL | ESR - 1 h | 45 mm |
| Lymphocytes | 1,800/µL | AST | 71 IU/L | ESR - 2 h | 61 mm |
| RBC | 4,370 × 103/µL | ALT | 20 IU/L | Ferritin | 373.9 ng/dL |
| Hb | 11.0 g/dL | LDH | 912 IU/L | Serum β2MG | 4.77 µg/mL |
| PLT | 421 × 103/µL | CK | 136 IU/L | Urine β2MG | 4.55 µg/L |
| Aldolase | 33.4 IU/L | sIL-2R | 3,338 U/mL | ||
| PT-INR | 1.03 | Amylase | 67 IU/L | IgG | 3354.7 mg/dL |
| APTT | 36.8 s | BUN | 11.6 mg/dL | IgA | 334.1 mg/dL |
| Fib | 250 mg/dL | Creatine | 0.22 mg/dL | IgM | 66.1 mg/dL |
| D-dimer | 5.7 mg/dL | Na | 137 mEq/L | KL-6 | 258 U/mL |
| AT III | 81% | K | 4.2 mEq/L | NTproBNP | 337.4 pg/mL |
| Cl | 99 mEq/L | ||||
| Anti-nuclear antibody | 1:640 | Anti-NXP2 antibody | + | ||
| Anti-dsDNA antibody | - | Anti-Jo-1 antibody | - | ||
| Anti-Sm antibody | - | Anti-ARS antibody | - | ||
| Anti-SS-A antibody | - | Anti-TIF1γ antibody | - | ||
| Anti-SS-B antibody | - | Anti-MDA5 antibody | - | ||
| Anti-CCP antibody | 0.8 U/mL | Anti-Mi-2 antibody | - | ||
| Anti-U1-RNP antibody | - | ||||