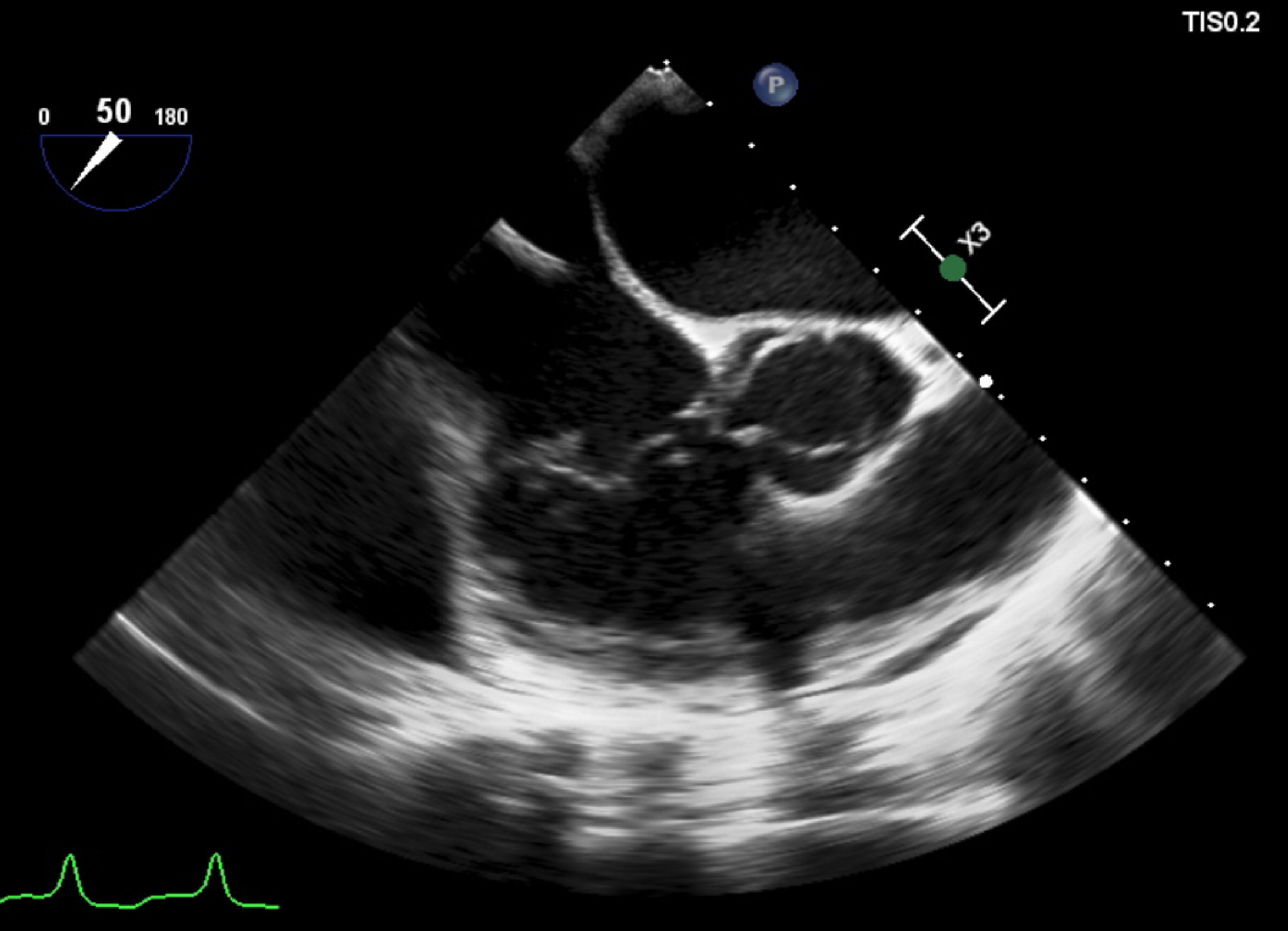
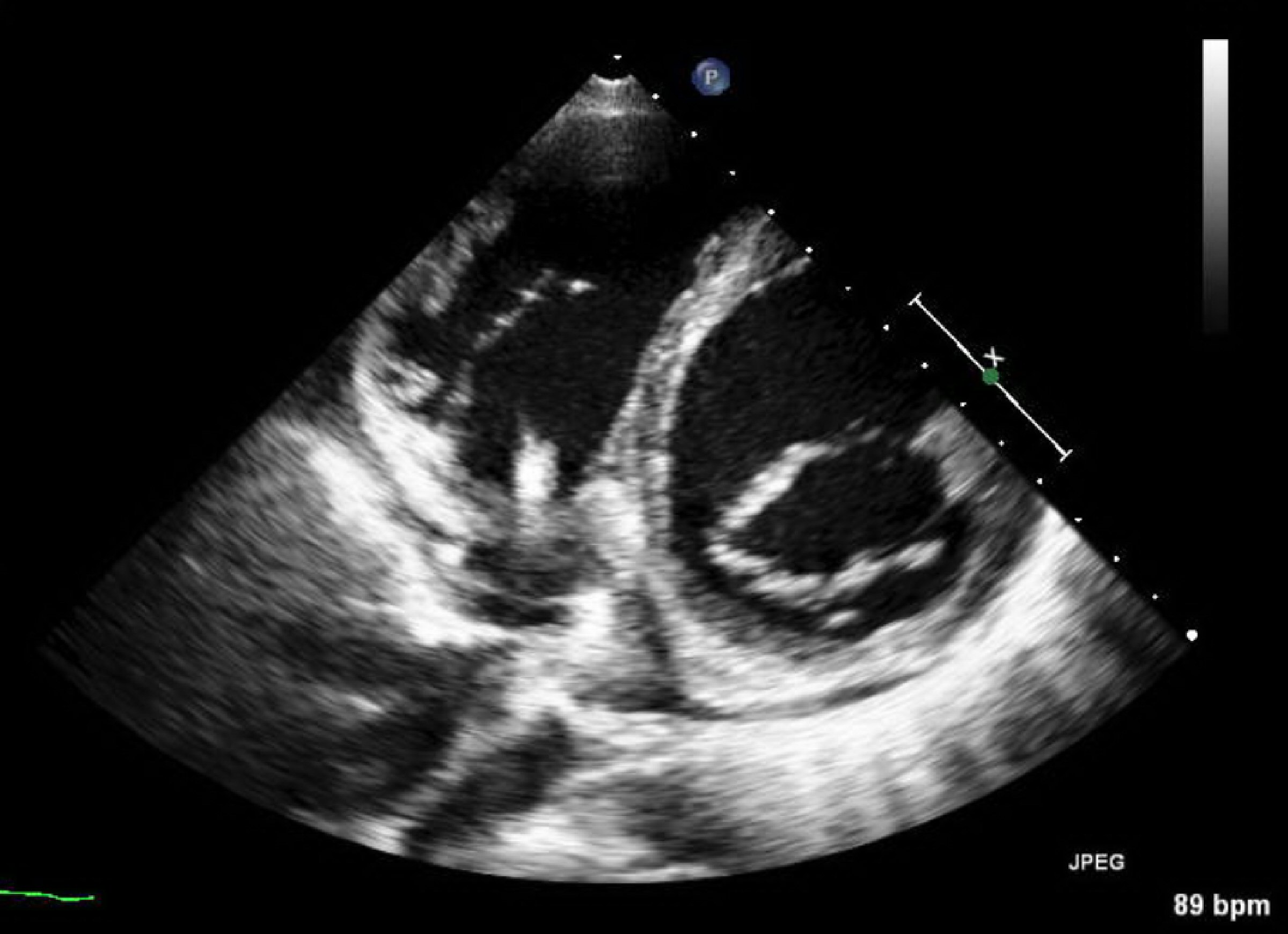
Figure 1. Transesophageal echocardiogram showing the vegetation adherent to the posterior leaflet of tricuspid valve. This exam was unremarkable for fibrin-sheath, masses or vegetations adherent to the electrocatheter.
| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 12, Number 2, February 2021, pages 61-64
Corynebacterium striatum Cardiac Device-Related Infective Endocarditis: The First Case Report in a Patient With a Cardiac Resynchronization Therapy Defibrillator Device and Review of the Literature
Figures

Table
| Ref. | Gender, age | Comorbidities | Cardiac devices | IE location (and other infected locations associated) | TTE and TTE findings | Cultures positive to C. striatum | Antibiotic susceptibility testing reveling sensitivity (MIC, in µg/mL) | Medical treatment | Surgery | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| BC: blood cultures; D: in-patient day; F: female; IE: infective endocarditis; M: male; MIC: minimum inhibitory concentration; ND: not described; Ref.: reference; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography. | ||||||||||
| [3] | M, 73 | ND | Pacemaker (6 years before, with battery replacement 2 years before without removal of old electrode wire) | Intracardiac lead and tricuspid valve (pacemaker sinus tract) | TTE: normal; TEE: vegetations on the old electrode wire | BC; drainage pus | Vancomycin (ND) | 1) Vancomycin (4 weeks); 2) Co-trimoxazole + rifampin (4 weeks); 3) Vancomycin (4 weeks) | Yes (1st intervention: removal of pacemaker battery; 2nd intervention: removal of old electrode wire) | Alive |
| [4] | F, 71 | ND | Pacemaker (replaced 2 months before) | Intracardiac lead (ND) | TTE: mobile mass adherent to the intracardiac lead | Device | Vancomycin (0.5)/linezolid (0.25)/daptomycin (0.125) | 1) Daptomycin 6mg/kg body weight (7 days); 2) Linezolid; 3) Daptomycin (4 weeks) | Yes (8th day, device removal, and reimplantation of a new pacemaker) | Alive |
| [5] | M, 51 | ND | Pacemaker (7 months before) | Pacemaker intraatrial lead (ND) | TEE: vegetations intracardiac wire | BC; device | Penicillin (ND)/gentamicin (ND)/tobramycin (ND)/erythromycin (ND)/lincomycin (ND)/linezolid (ND)/chloramphenicol (ND)/tetracycline (ND)/rifampicin (ND)/co-trimoxazole (ND)/ofloxacin (ND)/teicoplanin (ND)/vancomycin (ND) | 1) Vancomycin + ciprofloxacin (6 weeks) | Yes (device removal) | Alive |
| [6] | M, 78 | Diabetes, chronic renal failure | Pacemaker (6 months before) | Pacemaker intraventricular lead and tricuspid valve (spondylodiscitis D10 - D11 and epidural abscess) | TTE: mobile masses adherent to the intracardiac lead from the tricuspid valve to the right ventricular wall. | BC | Daptomycin (0.064)/vancomycin (0.36) | 1) Daptomycin 10 mg/kg | Yes (10th day removal of electrode wires followed by re-implantation of new wires 9 days later) | Alive |
| [7] | F, 79 | ND | Pacemaker (7 years before) | Pacemaker lead (ND) | TTE: normal; TEE: normal | BC; device | ND | 1) Vancomycin (6 weeks) | Yes (pacemaker removal due to dysfunction) | Alive |