Figures
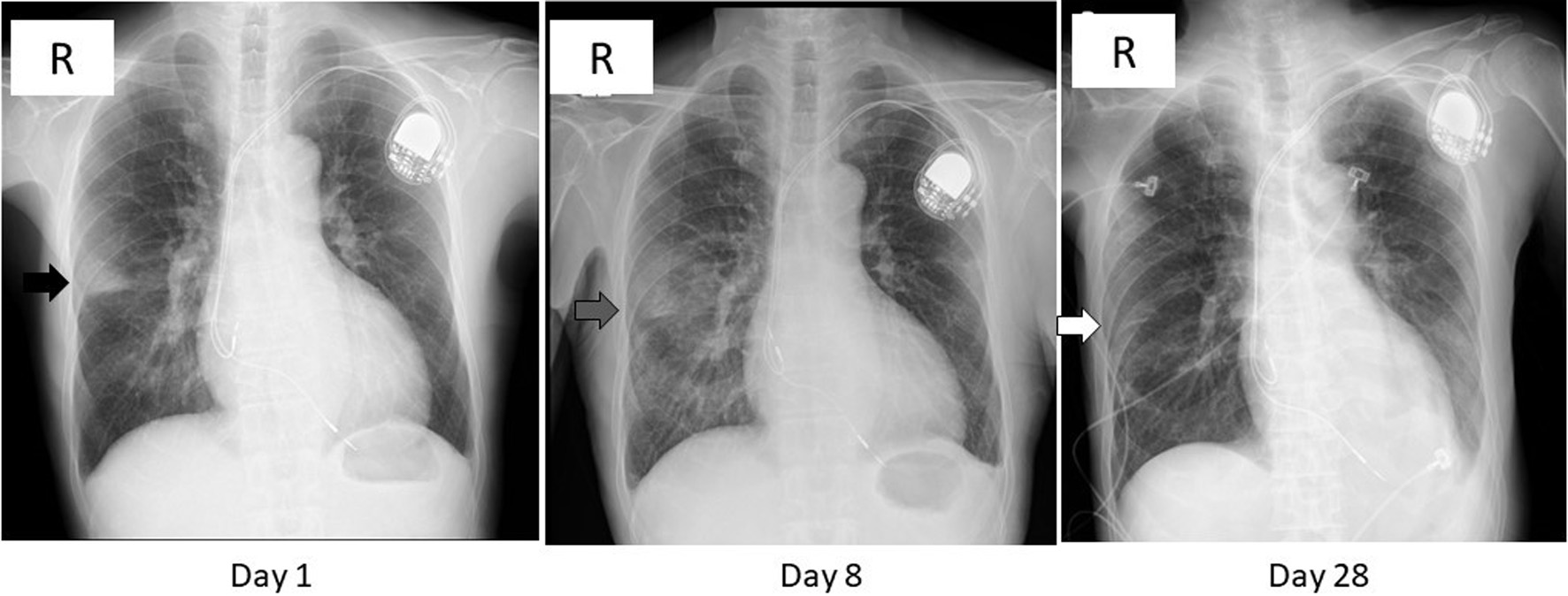
Figure 1. Chest X-ray images on day 1, 8, and 28. A wedge-shaped opacity with a relatively clear margin was observed in the right middle lung zone (black arrow) on day 1. That opacity turned blurred and extended in the lower lung field (gray arrow) on day 8, then diminished (white arrow) on day 28. The radiolucency of left lower lung field was reduced on day 28 due to pleural effusion (see Fig. 2).
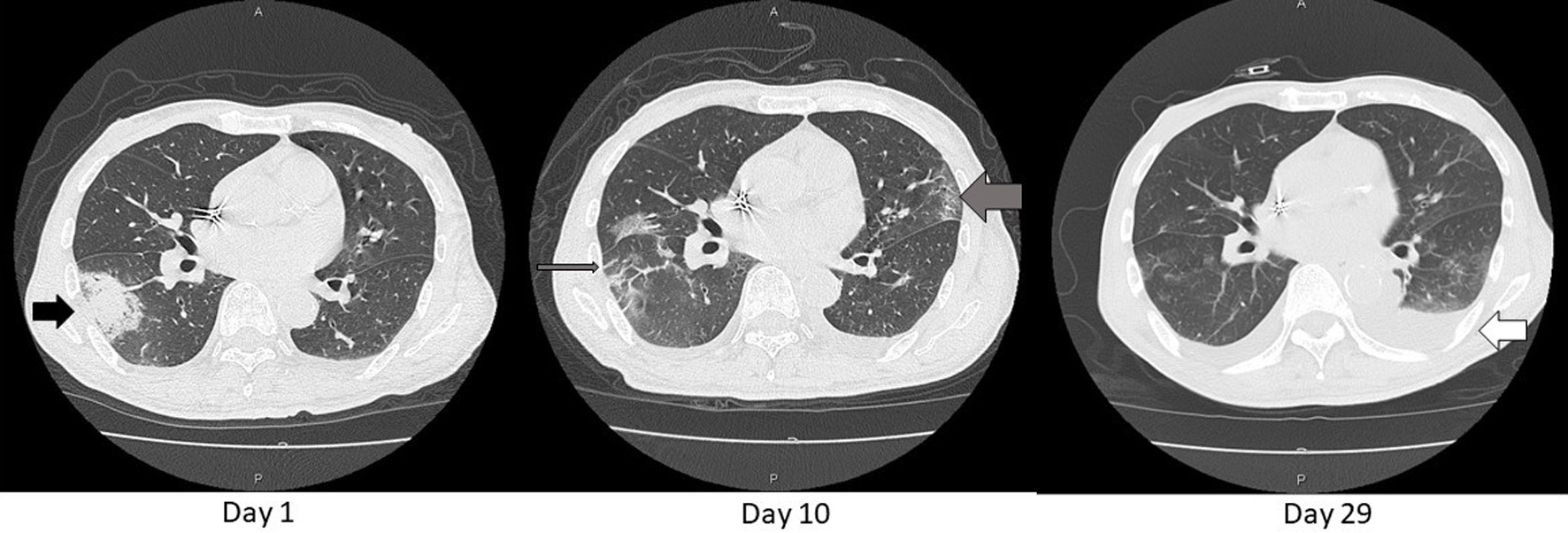
Figure 2. Axial chest CT images on day 1, 10, and 29. An opacity found in the chest X-ray (Fig.1) was consolidative in S6 of the right lung (black arrow) on day 1. That consolidation became blurred and extended as GGO (thin gray arrow) and similar shadows also appeared in the left lung (thick gray arrow) on day 10. These shadows diminished except for left pleural effusion on day 29 (white arrow). That effusion was gradually decreasing with adjustment of body fluid by dialysis sessions. CT: computed tomography; GGO: ground glass opacification.
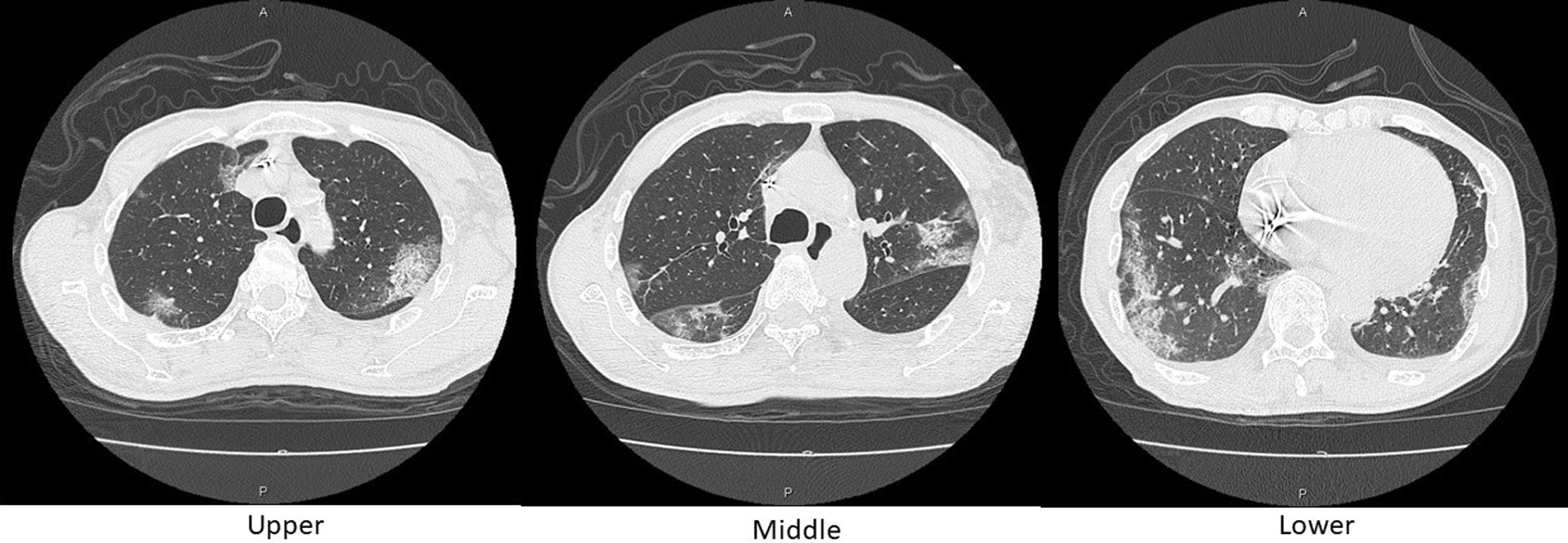
Figure 3. Axial chest CT images on day 10. Reticular shadows with a peripheral distribution, partially with crazy-paving appearance, scattered in the bilateral lungs. CT: computed tomography.
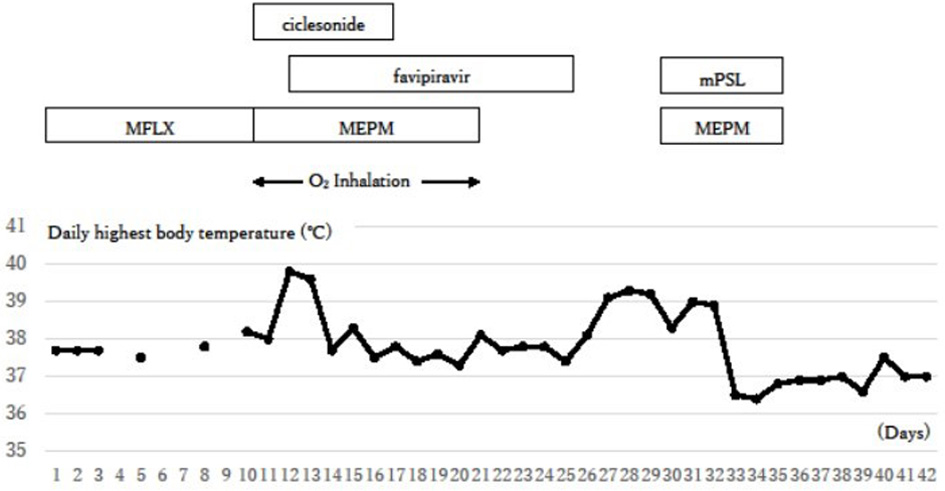
Figure 4. Clinical course. The inflammation signs were initially reduced with the treatments against COVID-19, but they flared up again around day 26. Additional steroid therapy against a possible cytokine storm succeeded in reducing symptoms. COVID-19: coronavirus disease 2019; O2 inhalation: oxygen inhalation of 1 - 2 L/min through a nasal cannula; MFLX: moxifloxacin; MEPM: meropenem; mPSL: methyl prednisolone.
Tables
Table 1. Laboratory Findings Before the Dialysis Session on Day 1
| Parameter | Value | Unit |
|---|
| AST: aspartate aminotransferase (GOT); ALT: alanine aminotransferase (GPT) |
| White blood cells | 4,630 | /µL |
| Neutrophils | 76.8 | % |
| Lymphocytes | 11.2 | % |
| Basophils | 0.4 | % |
| Eosinophils | 4.3 | % |
| Monocytes | 7.3 | % |
| Red blood cells | 353 | × 104/µL |
| Hemoglobin | 11.4 | g/dL |
| Hematocrit | 33.5 | % |
| Platelets | 7.3 | × 104/µL |
| Total protein | 6.6 | g/dL |
| Albumin | 3.8 | g/dL |
| Blood urea nitrogen | 73 | mg/dL |
| Creatinine | 9.46 | mg/dL |
| Na (Sodium) | 136.3 | mEq/L |
| K (Potassium) | 4.61 | mEq/L |
| Chloride | 96.3 | mEq/L |
| Calcium | 8.4 | mg/dL |
| Inorganic phosphorus | 4.5 | mg/dL |
| AST | 32 | U/L |
| ALT | 27 | U/L |
| Glucose | 215 | mg/dL |
Table 2. Parameters in Diagnosis of COVID-19
| Parameter | Unit | Day 1 | Day 5 | Day 8 | Day 10 | Day 12 | Day 15 | Day 18 | Day 21 | Day 25 | Day 28 | Day 30 | Day 32 | Day 35 | Day 42 |
|---|
| LDH: lactate dehydrogenase; IL-6: interleukin-6; CF: complement fixation test; CMV: cytomegalovirus; Ag: antigen; HRP: horseradish peroxidase; ELISpot: enzyme-linked immune absorbent spot; M. tuberculosis: Mycobacterium tuberculosis; RT-PCR: reverse transcription-polymerase chain reaction; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; IgM: immunoglobulin M. |
| Lymphocyte count | /µL | 519 | 509 | | 323 | 331 | | 259 | 428 | 478 | 389 | 259 | 421 | 400 | |
| LDH | U/L | | 276 | | 277 | 366 | | 309 | 258 | 241 | 213 | 248 | 240 | 229 | 180 |
| Glycoalbumin | % | | | | | | | | | | 38.1 | | | | 38.0 |
| C-reactive protein | mg/dL | 0.58 | 2.75 | | 2.00 | 7.21 | | 8.61 | 3.67 | 2.68 | 5.31 | 5.00 | 3.47 | 1.40 | 0.97 |
| Procalcitonin | ng/mL | | | | 0.89 | | | | | | | | | | |
| KL-6 | U/mL | 188 | | | | | | | | | | | | | |
| Fibrinogen | mg/dL | | 423 | | | | | | | | | 361 | 283 | 208 | |
| D-dimers | µg/mL | | 1.56 | | | | | | | | | 8.98 | 14.28 | 13.28 | |
| IL-6 | pg/mL | | | | | | | | | | 42.0 | | | | |
| Influenza A antibodies (CF) | × | | | | | | | | | 8 | | | | | 8 |
| Influenza B antibodies (CF) | × | | | | | | | | | < 4 | | | | | < 4 |
| CMV antibodies (CF) | × | | | | | | | | | 8 | | | | | 8 |
| CMVpp65Ag C7-HRP | +/- | | | | | | | | | | | | - | | |
| β-D-glucan | pg/mL | | | | 6.1 | | | | | | | 31.5 | | | |
| Cryptococcus neoformans antigen | +/- | | | | - | | | | | | | - | | | |
| Aspergillus antigen | +/- | | | | - | | | | | | | - | | | |
| ELISpot (for M. tuberculosis) | +/- | | | | - | | | | | | | | | | |
| RT-PCR for SARS-CoV-2 | +/- | - | - | - | - | | | - | | | | | | | |
| SARS-CoV-2 antibodies IgM | +/- | - | | | + | | + | | + | | | | | | |
| SARS-CoV-2 antibodies IgG | +/- | - | | | - | | + | | | | | | | | |
| Blood culture | +/- | | | | - | | | | | | | - | | | |



