| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 1, January 2013, pages 29-33
Autoimmune Pancreatitis Presenting as a Focal Pancreatic Mass Mimicking Pancreatic Cancer: A Case Report and Review of the Literature
JayaKrishna Chintanaboinaa, c, Bharat K. Patelb, Alexander T. Lalosb, Edward A. Sherwinb, David Ruttab, Christopher A. Barbarevechb
aWright Center for Graduate Medical Education, 501 Madison Ave, Scranton, PA 18510, USA
bGastrointestinal Consultants of NEPA, 517 Ash St Suite 1, Scranton, PA 18509, USA
cCorresponding author: JayaKrishna Chintanaboina, Wright Center for Graduate Medical Education, 501 Madison Ave, Scranton, PA 18510, USA
Manuscript accepted for publication September 24, 2012
Short title: Autoimmune Pancreatitis Presenting
doi: https://doi.org/10.4021/jmc891w
| Abstract | ▴Top |
A 68-year-old female presented with a 2-week history of jaundice, generalized itching and weight loss in 2006. On physical examination, she was deeply jaundiced; however, other clinical examinations were completely unremarkable. Liver function tests (LFTs) were deranged: serum alkaline phosphatase 407 U/L (35 - 104), serum aspartate transaminase 57 U/L (0 - 31), serum alanine transaminase 184 U/L (0 - 37), and serum total bilirubin 4.3 mg/dL (0 - 1). Abdominal CT revealed a 4.8 cm × 3.5 cm mass in the pancreatic head. ERCP revealed a high-grade stricture in the distal CBD. An internal biliary stent was placed in to the CBD stricture. Patient’s symptoms relieved after biliary stenting. Serum CA 19-9 was elevated at 1,568 U/mL (0 - 37). Due to high suspicion for pancreatic cancer, patient underwent pylorus-sparing pancreaticoduodenectomy. Histopathology was benign and showed perilobular lymphoplasmacytic infiltrate, periductal fibrosis and phlebitis that was consistent with autoimmune pancreatitis. Patient was started on oral prednisone that was eventually replaced with azathioprine. Serum IgG4 was 53 mg/dL (1 - 291) after starting steroid treatment.
Keywords: Pancreatic mass; Autoimmune; Pancreatitis; Cancer
| Introduction | ▴Top |
Autoimmune pancreatitis (AIP) is a form of chronic pancreatitis of autoimmune etiology that is increasingly encountered in the last decade. Although first described by Sarles et al [1] in 1961 as ‘Chronic inflammatory sclerosis of the pancreas’, it was first termed as ‘Autoimmune pancreatitis’ by Yoshita et al [2] in 1995. It rarely presents as a focal mass lesion in the pancreas, which can be commonly mistaken for a pancreatic cancer (PC). The following case describes an AIP patient who presented with clinical symptoms closely mimicking PC. We also discuss the potential scenarios of misdiagnosis of AIP and review the literature.
| Case Report | ▴Top |
A 68-year-old female with no significant past medical history presented with a 2-week history of jaundice and generalized itching in May 2006. She had lost about 15 lbs. She denied abdominal pain. She was recently diagnosed with type-2 diabetes mellitus (DM). She denied history of jaundice in the past. Family history was significant for Crohn’s colitis in her brother. She denied alcohol abuse. On physical examination, she was deeply jaundiced; however, other clinical examinations were completely unremarkable. There were no stigmata of chronic liver disease. Liver function tests (LFTs) were deranged: serum alkaline phosphatase 407 U/L (35 - 104), serum aspartate transaminase 57 U/L (0 - 31), serum alanine transaminase 184 U/L (0 - 37), and serum total bilirubin 4.3 mg/dL (0 - 1). Abdominal computed tomography (CT) revealed a 4.8 cm × 3.5 cm mass in the pancreatic head with secondary obstruction and dilation of the common bile duct (CBD), and intrahepatic ductal dilation (Fig. 1) Endoscopic retrograde cholangiopancreaticography (ERCP) revealed a high-grade stricture in the distal CBD, measuring 2 - 2.5 inches and proximal CBD dilation (Fig. 2). A 7-French 9 cm long plastic internal biliary stent was placed in to the CBD stricture (Fig. 3). Patient’s symptoms i.e., jaundice and pruritus relieved after biliary stenting. Brushings obtained from CBD stricture during ERCP was non-conclusive. Serum CA 19-9 was elevated at 1,568 U/mL (0 - 37); alpha-feto protein was 1.85 ng/mL (< 8.7); and carcinoembryonic antigen was 0.6 ng/mL (0 - 3). Due to high suspicion for PC due to markedly elevated serum CA-19-9 levels, patient underwent pylorus sparing pancreaticoduodenectomy (Whipple’s procedure) at a tertiary care center. Intraoperative findings included a diffusely firm pancreas with a significant enlargement of the head of pancreas and peripancreatic inflammation. She had an uneventful postoperative recovery. Histopathology was benign and showed perilobular lymphoplasmacytic infiltrate, periductal fibrosis and phlebitis that was consistent with AIP. Immunohistochemical staining was positive for lymphocyte markers. Patient was started on oral prednisone that was eventually replaced with azathioprine. Serum IgG4 was 53 mg/dL (1 - 291) after starting steroid treatment. Liver biopsy was performed to rule out autoimmune hepatitis due to persistently elevated liver enzymes. Biopsy revealed mild neutrophilic infiltration of the bile duct epithelium and mild expansion of the portal tracts. Bile ductules were normal and there was no evidence of lobular inflammation, which ruled out autoimmune hepatitis. Several weeks after surgery and initiation of steroid therapy, LFTs returned to normal and the patient remained clinically stable.
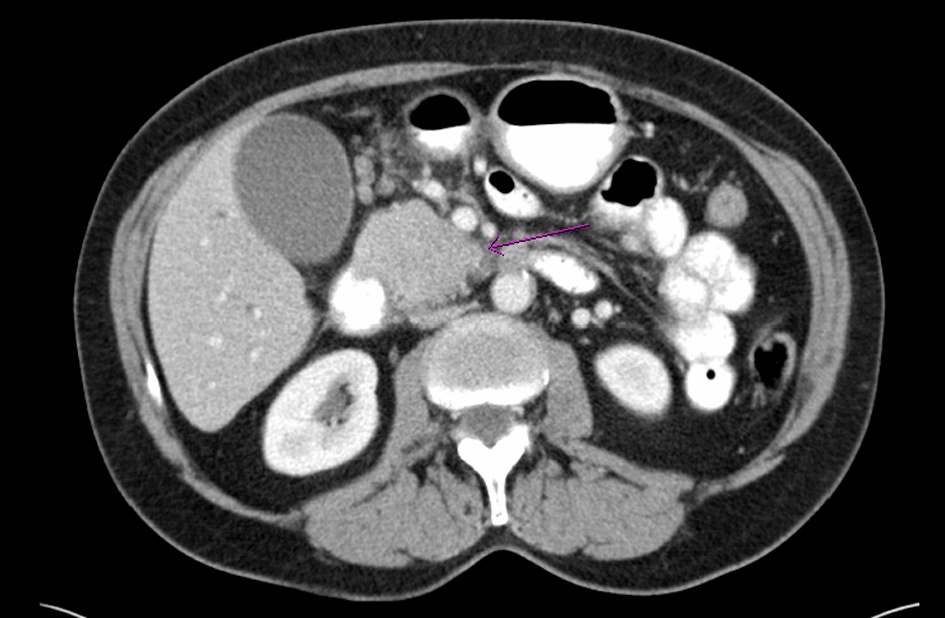 Click for large image | Figure 1. Computed tomography of abdomen shows a mass (arrow) in the head of pancreas |
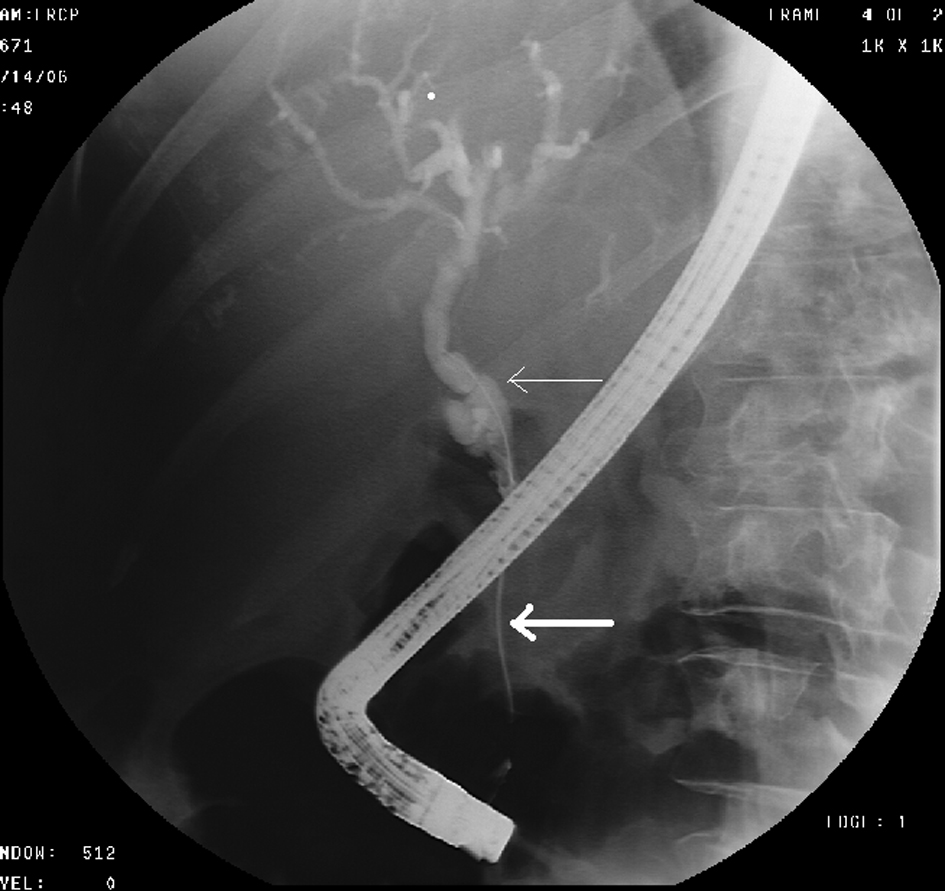 Click for large image | Figure 2. ERCP shows high-grade stricture (thick arrow) in the distal CBD and dilation of the proximal CBD (thin arrow). |
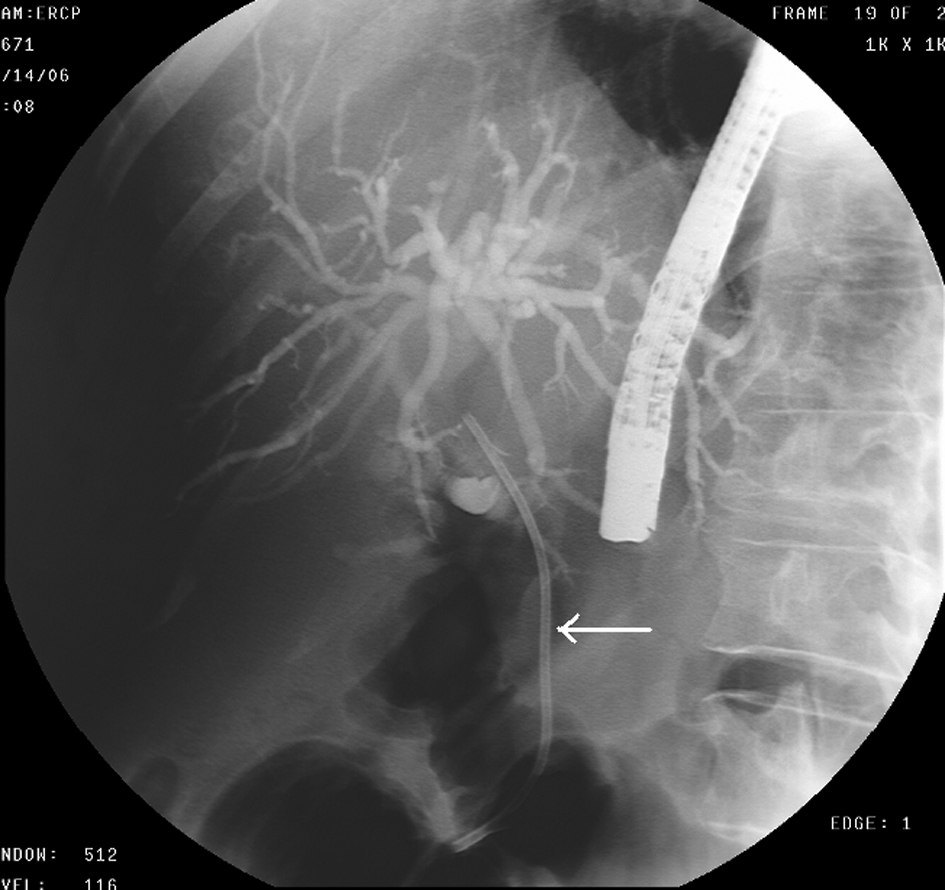 Click for large image | Figure 3. ERCP- Biliary stent (arrow) placed in to the stricture of CBD. |
| Discussion | ▴Top |
Although first described in 1961, the concept of AIP was strengthened in 2001 when Hamano et al. reported that serum IgG4 levels are specifically elevated in Japanese patients with AIP, which was later confirmed in Western and other Eastern countries [3-5]. Although AIP is considered more prevalent in Japan and other Asian countries; it is being increasingly diagnosed among western population in the last few years. This could be probably secondary to the increasing awareness and proposed diagnostic criteria. Approximately 3-5% of patients with AIP undergo pancreatic resection for presumed PC [6]. This subset of patients typically presents with clinical manifestations closely mimicking pancreatic cancer, as was seen in our patient.
Two different types of AIP are recently identified- type 1 or lymphoplasmacytic sclerosing pancreatitis (LPSP) and type 2 or idiopathic duct centric pancreatitis (IDCP) or granulocyte epithelial lesion (GEL) [7]. Type 1 is the classic form, which is associated with elevated serum IgG4 levels and tissue infiltration with IgG4+ plasma cells [8]. Type 2 is related to the neutrophilic infiltration to the pancreatic duct epithelium (GEL), and is not related to the IgG4 [9]. Type 1 is more predominant than type 2 and the exact prevalence of the latter is still not known. Hence, this review will only focus on type 1 AIP, which was diagnosed in our patient based on the histopathology.
The common clinical manifestations of AIP include midabdominal pain, obstructive jaundice, DM, anorexia and weight loss [10]. Less frequently, a persistent pancreatic mass, which is often confused with PC, as was seen in our patient, may be present. Common extrapancreatic manifestations include inflammatory bowel disease (primarily ulcerative colitis); lung nodules; retroperitoneal fibrosis; bile duct strictures; and other associated autoimmune conditions like autoimmune thyroiditis, autoimmune sclerosing cholangitis and Sjogren’s syndrome [11]. Although the most common pancreatic manifestations of AIP are also typically seen in patients with PC, however, extrapancreatic manifestations of AIP are less common in PC. A search on ‘pubmed’ revealed few case reports of AIP mimicking PC. Table 1 summarizes all the previously reported cases of AIP mimicking PC over the last decade.
 Click to view | Table 1. Summary of Previously Reported Cases of AIP Mimicking PC |
Serum IgG4 levels are significant when they are twice the upper limit of normal (> 280 mg/dL) as it is 99% specific for AIP at this level [11]. Frulloni L et al reported a novel serologic marker of AIP, antibodies against plasminogen-binding protein (Anti-PBP) peptide, which was present in 94% of AIP patients and in no health controls. However, in this study, these Anti-PBP peptide antibodies were also present in 5% of the patients with PC [12]. The most significant finding on an ERCP or magnetic resonance cholangiopancreatography (MRCP) includes a diffuse, irregular narrowing of the pancreatic duct; a narrowed main and dorsal pancreatic duct, or a focal stricture of the pancreatic duct [13]. The characteristic findings on CT scan, ultrasound or magnetic resonance imaging (MRI) include diffuse or focal pancreatic enlargement, peripancreatic capsule-like rim, enhancement at the late phase of contrast-enhanced images, and abnormal signal intensity on the MRI [14]. Endoscopic ultrasound (EUS) may provide supportive evidence. The predominant histological feature of type-1 AIP is infiltration of the periductal space with plasma cells, T-lymphocytes and obliterative phlebitis [15], which was seen in the histopathology of the pancreatic tissue of our patient.
The most widely accepted criteria for diagnosis of AIP include HISORt criteria and Asian diagnostic criteria. HISORt criteria comprise five cardinal features: Histology, Imaging, Serology, Other organ involvement and Response to corticosteroid therapy [21]. Asian criteria comprises of imaging, serological and histopathology of the pancreatic biopsy lesion [22]. Both HISORt and Asian criteria depend on histology, serology and imaging studies for the diagnosis of AIP. ERCP is mandated in the Asian criteria, but not in HISORt criteria. About 10% of patients with PC also have elevated IgG4 levels, which sometimes limits the use of this test to differentiate AIP and PC [23]. At times, serum IgG4 can be normal even in the presence of classic histologic features and tissue IgG4 staining [23]. AIP exquisitely responds to corticosteroid therapy and, as mentioned above, this response to corticosteroids is one of the HISORt criteria for the diagnosis of AIP. Steroid therapy may hasten recovery, decreases complications and delays relapses [24]. A trial of corticosteroids may be helpful in differentiating seronegative AIP from pancreatic cancer depending on the response of ductal dilation to steroids, 2 weeks after starting the therapy [25].
In patients with PC, transient symptom relief may occur during steroid trial when the inflammation surrounding the tumor resolves with steroids. An imaging study must be performed towards the end of steroid trial to confirm the resolution of pancreatic mass. The dosage, duration and taper regimens of AIP may vary with each patient. The standard recommended starting dose of prednisone is 30 - 40 mg/day (0.6 mg/kg/day) [26]. CT scan and serological tests can be performed for follow up of resolution of AIP while on steroid therapy.
Our case emphasizes that AIP should always be included in the differential diagnosis of a pancreatic mass and the relevant diagnostic work up should be performed. Diagnostic criteria such as HISORt and Asian criteria should be promptly used to help make a clinical diagnosis of AIP, distinguish it from PC, and prevent unnecessary surgery or delayed initiation of steroid therapy. Our patient was diagnosed with AIP by a surgical biopsy specimen in 2006, i.e. prior to the development of the HISORt and Asian diagnostic criteria. In patients with a high level of clinical suspicion for PC, but without a preoperative tissue diagnosis of malignancy, resection is still justifiable, offering the only hope for cure.
Conflicts of Interest
None of the authors report any conflicts of interest with this manuscript.
| References | ▴Top |
- Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688-698.
pubmed - Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40(7):1561-1568.
pubmed doi - Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344(10):732-738.
pubmed doi - Hirano K, Komatsu Y, Yamamoto N, Nakai Y, Sasahira N, Toda N, Isayama H, et al. Pancreatic mass lesions associated with raised concentration of IgG4. Am J Gastroenterol. 2004;99(10):2038-2040.
pubmed doi - Pezzilli R, Corinaldesi R. IgG4 as a serological marker of autoimmune pancreatitis: the latest news. JOP. 2004;5(6):531-533.
pubmed - Wolfson D, Barkin JS, Chari ST, Clain JE, Bell RH, Jr., Alexakis N, Neoptolemos JP. Management of pancreatic masses. Pancreas. 2005;31(3):203-217.
pubmed doi - Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the honolulu consensus document. Pancreatology. 2010;10(6):664-672.
pubmed doi - Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139(1):140-148; quiz e112-143.
pubmed - Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27(8):1119-1127.
pubmed doi - Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134(3):706-715.
pubmed doi - Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, Petersen BT, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7(10):1097-1103.
pubmed doi - Frulloni L, Lunardi C, Simone R, Dolcino M, Scattolini C, Falconi M, Benini L, et al. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med. 2009;361(22):2135-2142.
pubmed doi - Horiuchi A, Kawa S, Hamano H, Hayama M, Ota H, Kiyosawa K. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc. 2002;55(4):494-499.
pubmed - Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, Lauwers GY, et al. Autoimmune pancreatitis: imaging features. Radiology. 2004;233(2):345-352.
pubmed doi - Deshpande V, Gupta R, Sainani N, Sahani DV, Virk R, Ferrone C, Khosroshahi A, et al. Subclassification of autoimmune pancreatitis: a histologic classification with clinical significance. Am J Surg Pathol. 2011;35(1):26-35.
pubmed doi - Ooi K, Merrett N. Autoimmune pancreatitis in a patient presenting with obstructive jaundice and pancreatic mass. HPB (Oxford). 2004;6(2):126-127.
pubmed - Bartholomew SV, Zigman A, Sheppard B. Lymphoplasmacytic sclerosing pancreatitis presenting as a pancreatic head mass in a child: case report and management recommendations. J Pediatr Surg. 2006;41(5):e23-25.
pubmed doi - Efeovbokhan N, Makol A, Cuison RV, Minter RM, Kotaru VP, Conley BA, Chandana SR. An unusual case of autoimmune pancreatitis presenting as pancreatic mass and obstructive jaundice: a case report and review of the literature. J Med Case Rep. 2011;5:253.
pubmed - Lo RS, Singh RK, Austin AS, Freeman JG. Autoimmune pancreatitis presenting as a pancreatic mass mimicking malignancy. Singapore Med J. 2011;52(4):e79-81.
pubmed - Matsumoto I, Shinzeki M, Toyama H, Asari S, Goto T, Yamada I, Ajiki T, et al. A focal mass-forming autoimmune pancreatitis mimicking pancreatic cancer with obstruction of the main pancreatic duct. J Gastrointest Surg. 2011;15(12):2296-2298.
pubmed doi - Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol. 2007;42(Suppl 18):39-41.
pubmed - Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43(6):403-408.
pubmed doi - Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102(8):1646-1653.
pubmed doi - Lara LP, Chari ST. Autoimmune pancreatitis. Curr Gastroenterol Rep. 2005;7(2):101-106.
pubmed doi - Moon SH, Kim MH, Park DH, Hwang CY, Park SJ, Lee SS, Seo DW, et al. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut. 2008;57(12):1704-1712.
pubmed doi - Hirano K, Tada M, Isayama H, Yagioka H, Sasaki T, Kogure H, Nakai Y, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007;56(12):1719-1724.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.








