| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 8, Number 11, November 2017, pages 335-339
A 37-Year-Old Female With Abdominal Pain and Diarrhea: A Case of Idiopathic Hypereosinophilic Syndrome
Ivan E. Ramirez De Oleoa, Abigail Lofchiea, Mateo Mejia Saldarriagaa, b, Muhammad Perveza
aDepartment of Internal Medicine, Albert Einstein College of Medicine/Jacobi Medical Center, Bronx, NY 10461, USA
bCorresponding Author: Mateo Mejia Saldarriaga, Department of Internal Medicine, Jacobi Medical Center, 1400 Parkway, Bronx, NY, USA
Manuscript submitted March 26, 2017, accepted April 20, 2017
Short title: Abdominal Pain and Diarrhea
doi: https://doi.org/10.14740/jmc2813w
| Abstract | ▴Top |
We report a case of a 37-year-old female who presented with diffuse abdominal pain associated with dysphagia, weight loss, and history of chronic non-bloody diarrhea. Labs showed severe eosinophilia and esophagogastroduodenoscopy (EGD) showed patchy erythema in antrum and body of the stomach. The pathological exam showed lymphoplasmacytic inflammation of lamina propria with increased eosinophil count and eosinophilic abscesses. The immunophenotypic analysis did not show evidence of clonality. There was no evidence of allergic reaction or infection. In view of the absence of causes to explain severe eosinophilia, the diagnosis of hypereosinophilic syndrome (HES) was made and she was started on steroids with improvement in her symptoms and eosinophils counts in subsequent evaluations.
Keywords: Hypereosinophilic syndrome; Eosinophilia; Strongyloides; FIP1L1-PDGFRA; Eosinophilic gastroenteritis; Bowel thickness
| Introduction | ▴Top |
Hypereosinophilic syndrome (HES) is characterized by sustained overproduction of eosinophils, leading to multi-organ damage by eosinophilic infiltration. HES is defined as the absolute count of eosinophils > 1,500 cells/µL on two separate occasions or pathologic confirmation of tissue hypereosinophilia in the absence of alternative etiologies. It is a rare disorder with estimated prevalence of 0.36 - 6.3 per 100,000 [1], and approximately one-third of these present with gastrointestinal (GI) symptoms [2]. We report a rare case of idiopathic HES associated with GI symptoms.
| Case Report | ▴Top |
A 37-year-old female presented to the emergency department with 3 weeks of diffuse abdominal pain. The patient reported 2 weeks of vomiting and 1 week of diarrhea 2 - 3 times per day. She also complained of difficulty swallowing and sensation of food stuck in her throat along with decreased appetite and weight loss of 10 pounds since illness started.
The patient’s medical history was remarkable for hypothyroidism and eosinophilia first noticed 8 years ago, for which she underwent multiple diagnostic tests including bone marrow examination, positive for marked eosinophilia, negative for granulomas or tumor, evaluation for FIP1L1-PDGFRA mutation, and a thorough infectious workup. She was found positive for Strongyloides IgG and received a course of ivermectin with transient symptomatic improvement. She was re-admitted for the same symptoms 1 year ago, at which point she was negative for Strongyloides antibodies, but showed temporary improvement after a second course of ivermectin.
On the present admission, her vital signs showed BP of 118/70 mm Hg, RR of 18, HR of 83 bpm, and O2 of 98%. The physical exam was significant for mild abdominal tenderness to palpation diffusely and was otherwise unremarkable. Initial laboratory work was significant for WBC of 24,800 cells/µL with absolute eosinophil count of 13,144 cells/µL, Hgb of 14.8 g/dL, and Plt of 269,000/µL. TSH, free T4, and basic metabolic panel including phosphate, magnesium and liver enzymes were all within normal limits. Additional laboratory tests for stool O&P, Strongyloides ab, Toxocara ab, C. Diff, HIV, UA, ANA, lipase, ANCA-C, ANCA-P, dsDNA, SCL-70, anti-centromere, tryptase, C-reactive protein, and sedimentation rate were normal. Because she had responded to ivermectin in the past, she was treated with a third empiric course of ivermectin without improvement.
CT scan of the abdomen showed findings consistent with a long segment of small bowel wall thickening, greatest in the jejunum, consistent with enteritis. There was diffuse mild small bowel ileus with mild circumferential wall thickening in the visualized distal esophagus and a small volume of abdominal and pelvic ascites (Figs. 1 and 2). A small to moderate left pleural effusion was also seen (Fig. 1).
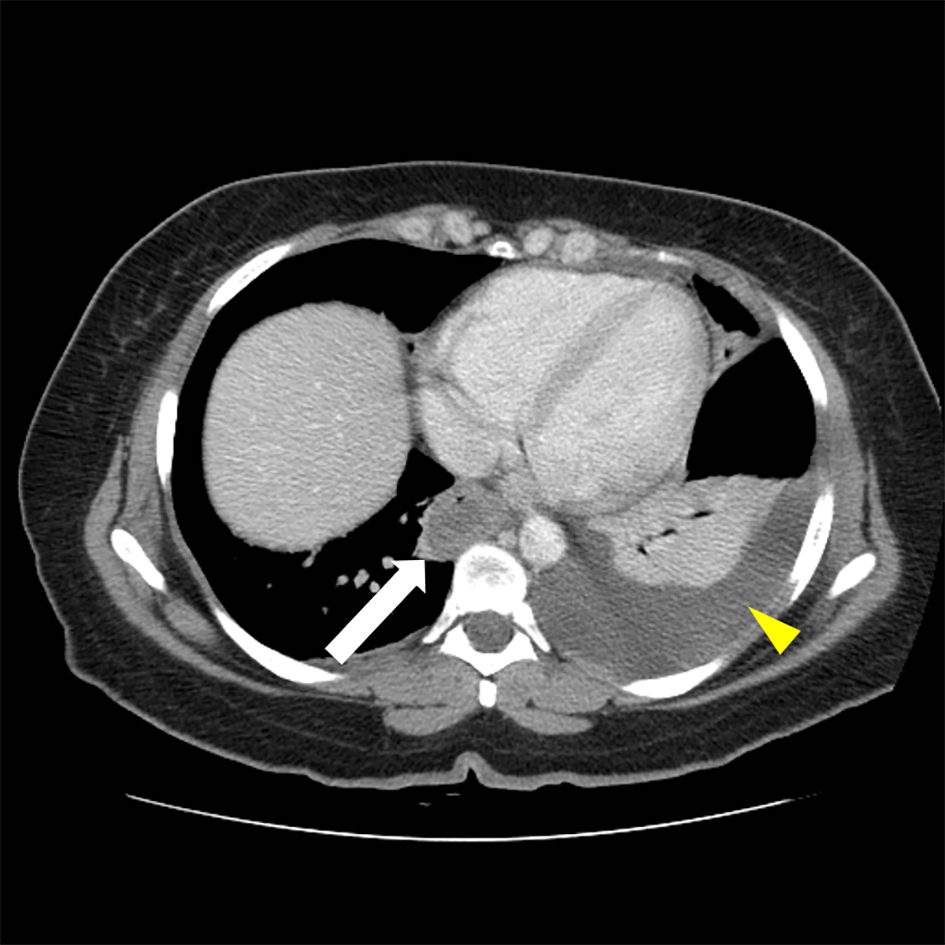 Click for large image | Figure 1. Abdominal CT, thickening of distal esophagus (white arrow) is evident. A left-sided pleural effusion is also seen (yellow arrow head). |
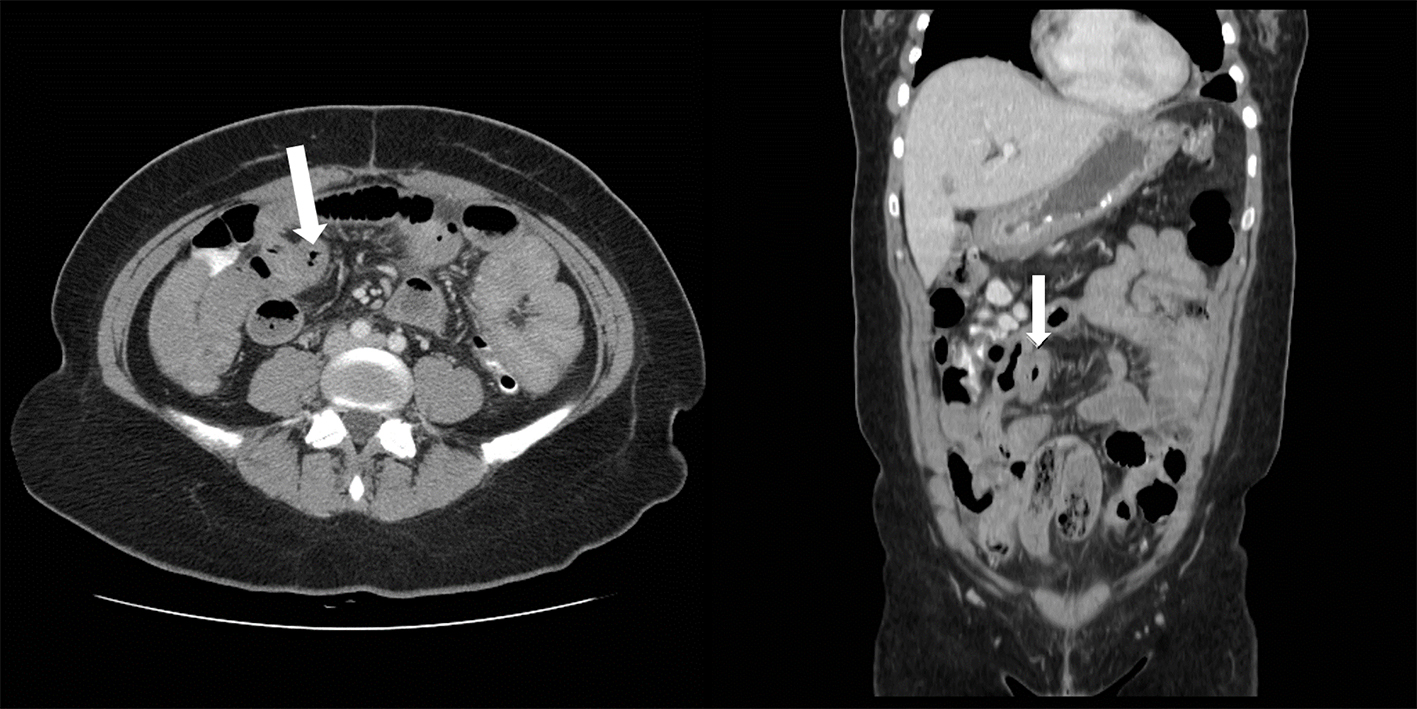 Click for large image | Figure 2. Abdominal CT, small bowel thickening (arrow) is evident in both axial (right) and coronal images. |
Esophagogastroduodenoscopy (EGD) was performed showing white plaques throughout the esophagus that washed off with water. A diffuse erythematous mucosa with mosaic pattern was found in the body of the stomach. Pathological examination reported esophageal mucosa with acute neutrophilic and eosinophilic esophagitis, eosinophils were focally too numerous to count and focal basal eosinophilic abscess was noted (Fig. 3). Gastric antrum and body-type mucosa showed evidence of chronic gastritis with mild lamina propria eosinophils (0 - 5 per high power field (HPF)) and focal stromal fibrosis with > 40 eosinophils per HPF. Duodenal mucosa revealed eosinophilic duodenitis with diffuse lamina propria eosinophils (Fig. 4).
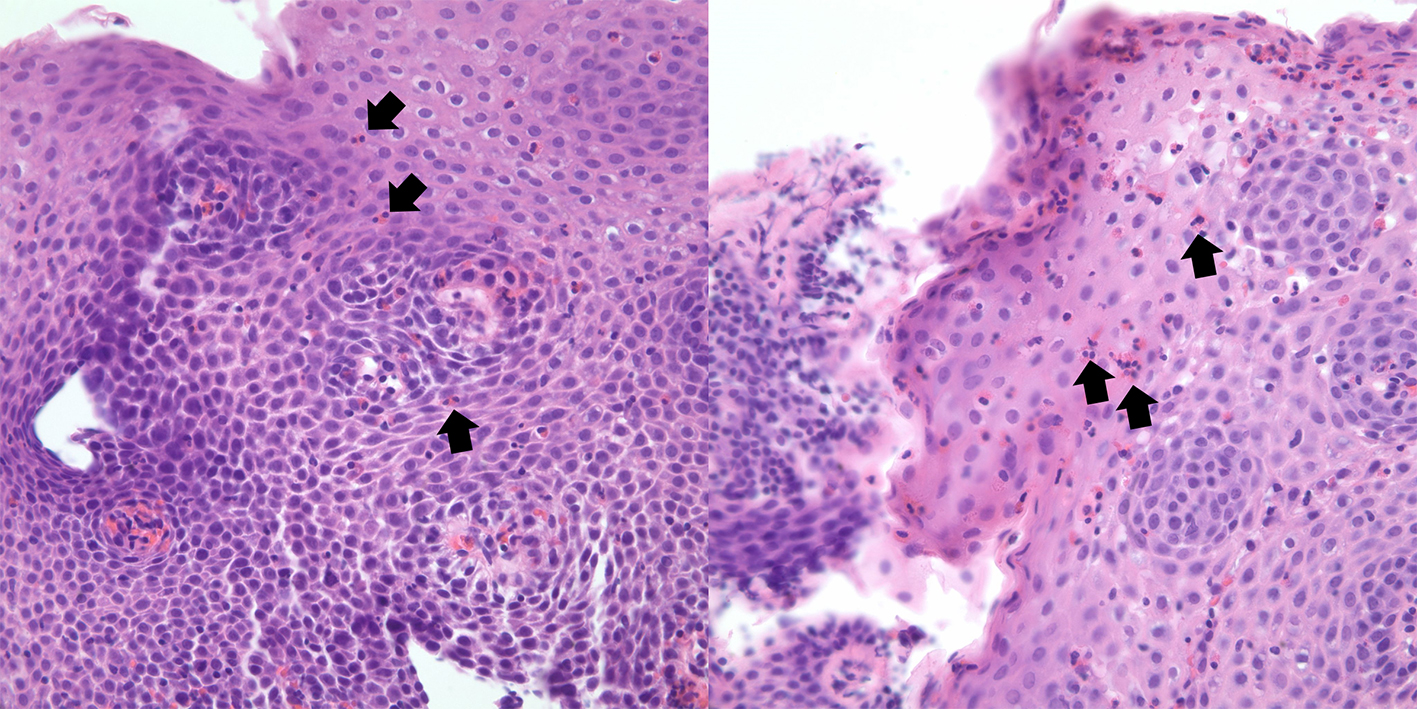 Click for large image | Figure 3. Hematoxylin and eosin stains of proximal (right) and distal (left) esophagus. Abundant eosinophils present (arrow), with the formation of eosinophilic abscess. |
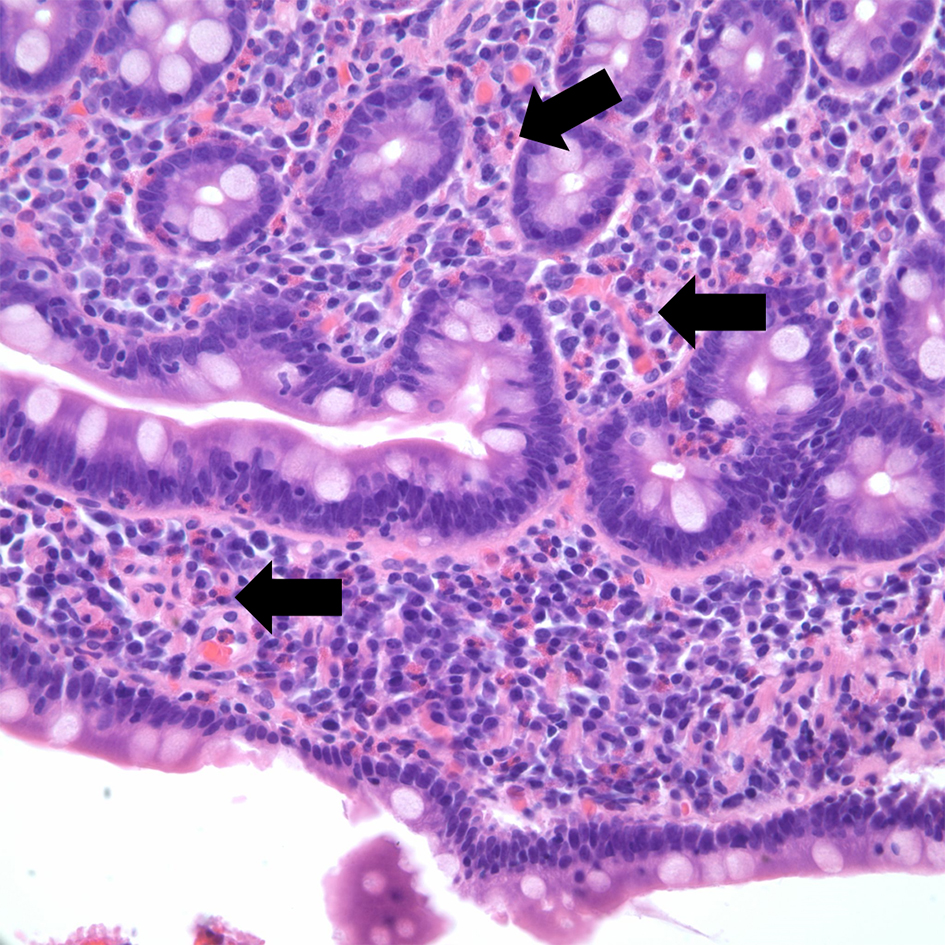 Click for large image | Figure 4. Hematoxylin and eosin stain of duodenal mucosa. Eosinophilic duodenitis with diffuse lamina propria eosinophils (arrow). |
An immunophenotypic analysis of peripheral blood showed eosinophilia and no evidence of a B-cell or T-cell lymphoma or signs of acute leukemia. In view of the absence of causes to explain the degree of eosinophilia, the diagnosis of HES was made. The patient was started on prednisone 60 mg daily and budesonide BID by nebulization with improvement in diarrhea and abdominal pain. On the 10th day of her hospital course, the patient was discharged home. A complete blood count on discharge showed a significant decrease in WBC with return of eosinophils to normal proportions.
The patient was seen in the clinic 7 days after discharge. She reported being well with minor abdominal pain. On physical examination, there was still mild abdominal tenderness to palpation. Prednisone was tapered to 50 mg daily. Complete blood count the day before the visit showed sustained suppression of eosinophils with a WBC of 11.4 cells/µL, absolute eosinophilic count of 0.9 cell/µL, Hgb of 12.7 g/dL, and Plt of 381,000/µL.
| Discussion | ▴Top |
The differential diagnosis of eosinophilia (defined as an absolute eosinophil count (AEC) > 500 cells/µL [3]) is broad and includes a wide range of conditions, from allergic to autoimmune disease (Table 1) [2, 4, 5]. The first step in the approach of eosinophilia is distinguishing between primary (where the main defect is affecting the regulation of eosinophils production) and secondary (or reactive) eosinophilia.
 Click to view | Table 1. Etiology of Eosinophilia |
To identify the etiology of the eosinophilia, a complete and thorough history is required. Some essential aspects include different signs and symptoms that are suggestive of end-organ compromise by eosinophilia (skin, pulmonary, cardiac, GI, skin, and neurologic), a complete medications and natural supplements list, past medical history including neoplastic conditions, allergic/atopic symptoms, travel and social history, and a complete physical examination to identify features suggestive of a specific disorder or etiology [5]. Medications are a frequent cause of eosinophilia, and the development of eosinophilia may not coincide with the introduction of the medications. Therefore, all medications taken by the patient should be evaluated as possible causes of eosinophilia. Allergy is also a common cause of eosinophilia; however, as a rule of thumb, it should not cause an AEC of > 1,500 cells/µL [2]. Other important etiologies are infections, most of which are parasitic. Strongyloides spp infection is common in patients from endemic regions (tropical/warm regions) or exposed to soil or waters contaminated with feces. Identification of Strongyloides spp is essential given the risk of hyperinfestation syndrome if the patient is started on immunosuppressive therapy for presumed immune-related causes of eosinophilia. Other causes of infectious eosinophilia include helminth infections such as Sarcocystis hominis, Toxocara spp, Schistosoma spp and viral infections including EBV and HIV infection [5].
In cases where no secondary cause of eosinophilia is identified, a diagnosis of HES should be considered. HES is a heterogeneous syndrome described by Chusid et al in 1975 [6] after a cohort of patients with unexplained eosinophilia and end-organ damage. After multiple classifications and diagnostic criteria, the definition was refined by Simon et al [7] in 2010 to include some variation of this syndrome and provide a functional approach (as opposed to World Health Organization classification, where a merely descriptive approach is used [3]). Currently, HES is defined has peripheral eosinophilia > 1,500 cells/µL in two different occasions or evidence of tissue hypereosinophilia with symptoms and peripheral eosinophilia after excluding secondary causes [7]. HES is divided into six sub-types [7]: 1) myeloproliferative HES (M-HES), where patients have features that resemble myeloproliferative neoplasm (such as cytopenia, spleno/hepatomegaly, elevated B12, and with a higher incidence in male population) often with monoclonal eosinophilia with distinct mutations or fusion proteins such as fusion gene PDGFRA-FIP1L1; 2) lymphocytic HES (L-HES) where a monoclonal T-cell population is often identified, and leads to eosinophilia through cytokine (usually IL-5) production, presenting with frequent skin compromise; 3) idiopathic HES, with no clear triggers found; 4) overlap HES, where only one system is affected and peripheral eosinophilia is present (eosinophilic GI disease, chronic eosinophilic pneumonia, among others); 5) associated HES, where a disorder known to have eosinophilia is found (such as HyperIgE syndrome, sarcoidosis and inflammatory bowel disease; and 6) familiar HES, where multiple generations are affected and usually present after birth [8]. This subdivision not only has a functional purpose, but also represents different phenotypes, different prognosis, and different therapeutic implications [9].
Conclusion
The present case represents a young woman with long-standing severe hypereosinophilia and multiple GI complains including vomiting, diarrhea and abdominal pain. Although this was initially thought to represent Strongyloides spp infection, the patient was seronegative at the time of presentation and did not respond to treatment. Peripheral blood flow cytometry did not identify any clonal subset of T cells or immature precursors, and other myeloproliferative features were absent. The patient responded well to initial systemic steroid management and was later tapered with no recurrence of symptoms or eosinophilia. The patient’s presentation and response to corticosteroids were consistent with idiopathic HES.
Conflict of Interest
No conflict of interest exists from any of the authors.
| References | ▴Top |
- Crane MM, Chang CM, Kobayashi MG, Weller PF. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J Allergy Clin Immunol. 2010;126(1):179-181.
doi pubmed - Curtis C, Ogbogu P. Hypereosinophilic Syndrome. Clin Rev Allergy Immunol. 2016;50(2):240-251.
doi pubmed - Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol. 2014;89(3):325-337.
doi pubmed - Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2015;2015:92-97.
doi - Kovalszki A, Weller PF. Eosinophilia. Prim Care. 2016;43(4):607-617.
doi pubmed - Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975;54(1):1-27.
doi - Simon HU, Rothenberg ME, Bochner BS, Weller PF, Wardlaw AJ, Wechsler ME, Rosenwasser LJ, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(1):45-49.
doi pubmed - Curtis C, Ogbogu PU. Evaluation and differential diagnosis of persistent marked eosinophilia. Immunol Allergy Clin North Am. 2015;35(3):387-402.
doi pubmed - Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, Kutok J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201-1214.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.








