| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 7, Number 9, September 2016, pages 379-383
A Case of Transient Loss of Vision Following Coronary Angiography: Etiology, Investigation and Management
Elizabeth McElneaa, d, David Gallagherb, Khaldoon Al-Tahsa, Thomas Kiernanc
aDepartment of Ophthalmology, University Hospital Limerick, Limerick, Ireland
bDepartment of Ophthalmology, University College Hospital Galway, Galway, Ireland
cDepartment of Cardiology, University Hospital Limerick, Limerick, Ireland
dCorresponding Author: Elizabeth McElnea, Department of Ophthalmology, University Hospital Limerick, Limerick, Ireland
Manuscript accepted for publication July 19, 2016
Short title: Loss of Vision After Coronary Angiography
doi: http://dx.doi.org/10.14740/jmc2591w
| Abstract | ▴Top |
Cortical blindness is, thankfully, a rarely encountered complication of coronary angiography. We present the case of a 72-year-old Caucasian gentleman in whom bilateral visual loss occurred abruptly after exposure to contrast during diagnostic coronary angiography. Areas of acute cerebral infarction were not appreciated at initial cranial computed tomography. Leakage of contrast medium into the occipital cortices was similarly absent. The patient recovered vision within 24 hours. Given the frequency with which coronary angiography is performed worldwide, an awareness of the causes of cortical blindness following the same is important. Although already well elaborated in the literature related to both cardiology and radiology, there are few reports in the general medical or ophthalmology literature that describe transient cortical blindness after coronary angiography and detail contrast-associated visual loss.
Keywords: Cortical blindness; Coronary angiography; Contrast
| Introduction | ▴Top |
The incidence of cerebrovascular complications including cerebrovascular accidents and transient ischemic attacks such as amaurosis fugax following coronary angiography is low. The British Cardiac Society has reported an incidence of 0.06% [1] and the National Institutes of Health in the United States of America that of 0.03% [2]. These are thus rare complications, considering the widespread, frequent, performance of coronary angiography.
Cortical blindness is better recognized as a complication of cerebral and in particular vertebral angiography having an incidence thereafter of 0.3-2.6% [2] but has also already been described after coronary angiography. Fischer-Williams et al reported one case in 12,367 coronary angiographies, an incidence of 0.008% [3] and Kinn et al reported three cases in over 6,000 such procedures, an incidence of 0.05% [4].
An idiosyncratic neurotoxic effect of the contrast media used has been implicated in some cases. Indeed, intra-ventricular, subarachnoid and brain parenchymal contrast enhancements have all been described following coronary angiography [5, 6]. A contrast media-related complication rate of 0.4% has been reported in patients who underwent coronary angiography wherein iopamidol was used as the contrast agent [7].
| Case Report | ▴Top |
A 72-year-old Caucasian gentleman underwent elective diagnostic cardiac catheterization prior to general anesthesia for transurethral resection of his prostate.
This man had a history of benign prostatic hypertrophy and additionally hypertension and atrial fibrillation. His medications at this time were spironolactone 25 mg, allopurinol 100 mg, tolterodine tartrate 2 mg, olmesartan 40 mg, lercanidipine 20 mg, doxazosin 4 mg, nebivolol 7.5 mg, warfarin 6 mg and atorvastatin 10 mg.
Pre-medication was with diazepam 5 mg orally. The procedure was performed without difficulty from the right radial artery using Judkins catheters (JL4 + JR4) introduced through a 6-French arterial hemostatic sheath. During the procedure, the catheters were repeatedly flushed with heparinized saline (concentration 4,000 IU/L of normal saline). Coronary angiography, left ventriculogram and an aortogram were performed. The procedure required 170 mL of the non-ionic, low-osmolar contrast agent omnipaque 350 (350 mg iodine/mL) containing iohexol. This was the patient’s first exposure to contrast medium. The procedure lasted approximately 15 min during which the patient remained hemodynamically stable.
Coronary angiogram revealed non-obstructive atheroma. Left ventriculogram showed normal left ventricle function and mild-moderate concentric left ventricular hypertrophy consistent with hypertension. Aortogram revealed a dilated aortic root and moderate aortic incompetence.
Four hours following coronary angiography, this gentleman complained of bilaterally reduced vision and hearing. At ophthalmological examination, this gentleman could perceive only light bilaterally. This gentleman had an anterior chamber, iris-fixated intraocular lens in his left eye. Nonetheless, pupil reactions were normal bilaterally. Extra-ocular movements were normal. Further, no abnormalities were noted at dilated fundoscopy.
Cranial computed tomography (CT) was performed to exclude an intracerebral hemorrhage. No additional contrast material was used during this investigation. Obtained 2 h after the onset of symptoms, this did not show an intracerebral bleed or infarct. Contrast enhancement of the occipital lobes was also absent. Similarly magnetic resonance imaging (MRI) of the brain showed no acute findings. Renal function tests were also normal. Holter monitoring confirmed the occurrence of atrial fibrillation with pauses the longest being 90 - 100 ms. Hemodynamically significant stenosis was absent at Doppler ultrasound of the carotid arteries.
A presumptive diagnosis of emboli to the posterior cerebral circulation was made and aspirin 300 mg was administered orally.
Over the following day, the patient’s vision gradually returned so that by the following evening, no gross visual deficit could be detected. Three days later, an episode of expressive dysphagia prompted repeat MRI of the brain. As Figure 1 shows, punctate areas of restricted diffusion on diffusion weight inversion images suggestive of areas of acute infarction likely related to emboli were identified in the occipital lobes bilaterally. These were much more obvious compared to those at the previous examination. An old infarct in the right occipital cortex was also noted.
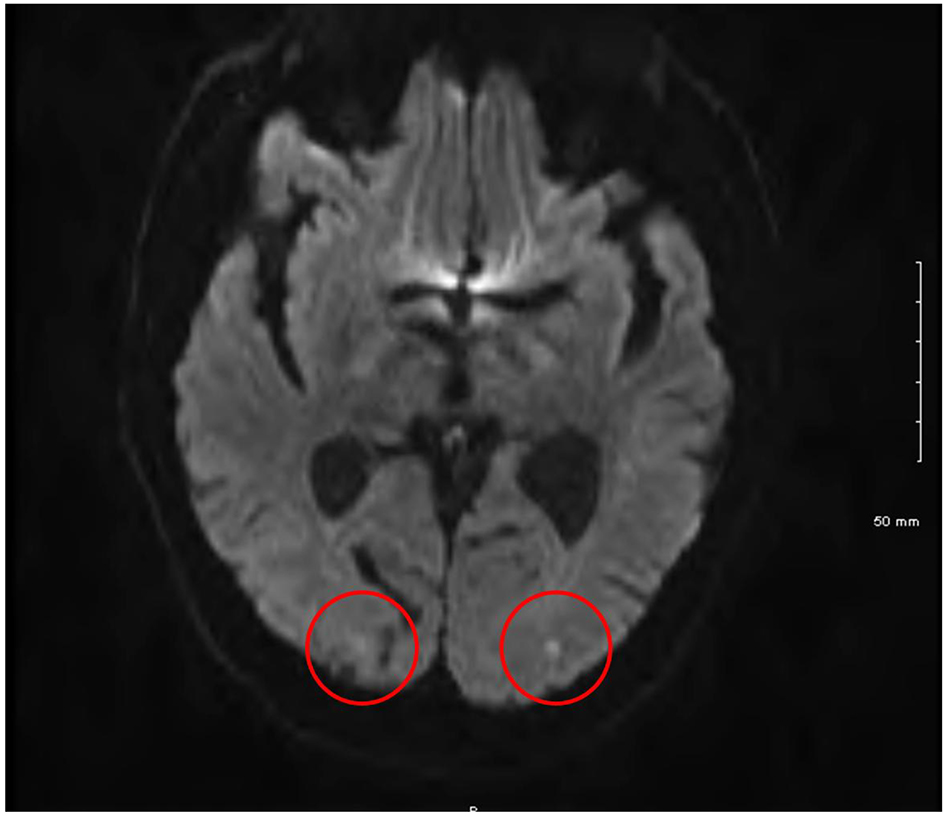 Click for large image | Figure 1. Cranial magnetic resonance imaging showing bilateral occipital lobe infarcts. |
The patient’s subsequent hospital stay was uneventful and he was discharged with no subjective residual neurological deficit. As the visual fields in Figure 2 show, small para-central visual field defects were demonstrable bilaterally at his 3-month follow-up visit.
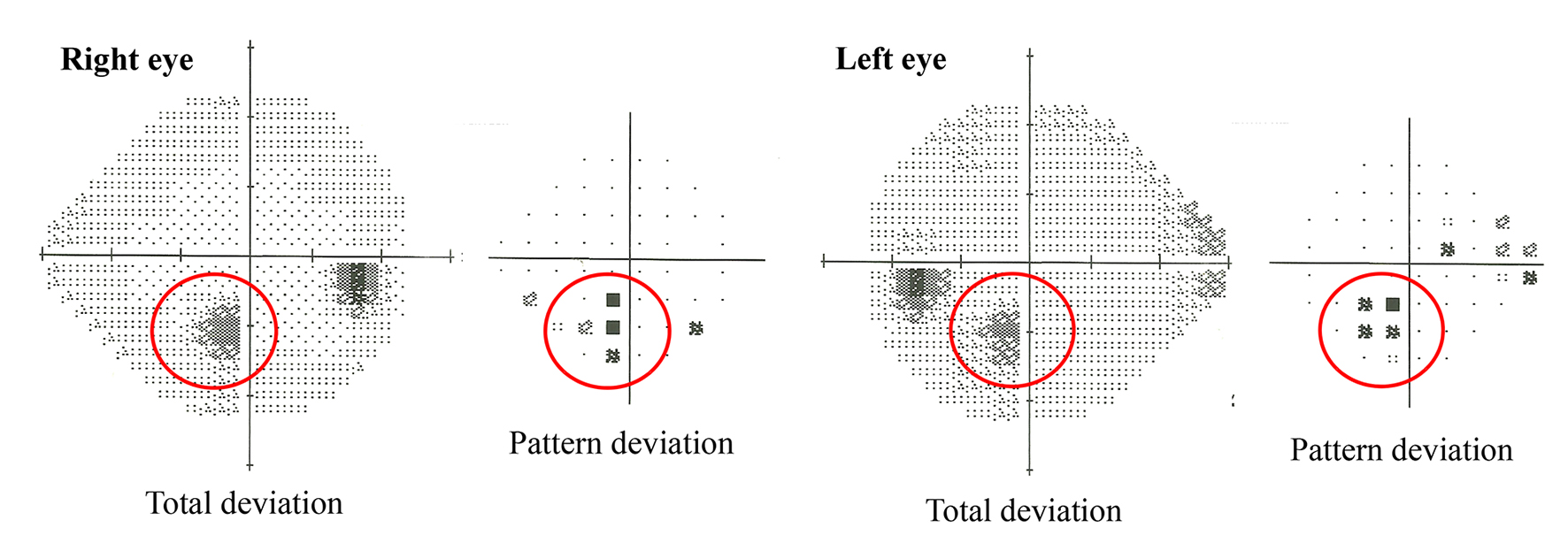 Click for large image | Figure 2. Humphrey 24/2 visual field testing revealed the presence of small para-central visual field defects bilaterally. |
| Discussion | ▴Top |
Differential diagnosis
In the case, we have described transient cortical blindness likely resulting from the dissemination of micro-emboli, from thrombus or atheromatous plaque, with the contrast agent, to the brain, while the right subclavian artery and/or brachiocephalic trunk was being crossed by the catheters employed in the procedure.
Micro-embolization has been reported in up to 5% of patients undergoing cardiac catheterization via the trans-brachial approach. Importantly, this frequency compares favorably with that observed when cardiac catheterization is performed via a femoral approach [8, 9].
Guide wires and catheter tips form foci for thrombus formation. Contact of the first of these with blood is particularly thrombogenic [10]. The formation of thrombi on the inner catheter surface is related to the duration of the procedure and may be associated with an increasing amount of blood refluxing into the catheter as the procedure time increases [11]. The procedure was not unduly prolonged in the case we describe here.
Guide wires and catheters may also mechanically disrupt atheromatous plaques. Again, manipulation of the guide wire in the case we have outlined could not be considered to have been excessive.
Non-ionic contrast lacks the anticoagulant activity of ionic contrast, but the incidence of thrombotic complications with both agents has been found to be similar [12].
Diffusion-weighted MRI is thought to be a useful way to detect even very small focal areas of brain ischemia in these settings [8], but as the case described here highlights, such areas may not always be readily apparent.
Contrast-induced transient cortical blindness is a rare entity, the pathophysiology of which remains largely speculative. The mechanism may involve an idiosyncratic toxic reaction which sees the contrast agent penetrate the brain parenchyma as a result of an acute disruption of the blood-brain barrier [13].
Contrast media are sterile iodine-containing solutions used in diagnostic imaging procedures. They are categorized into four groups: ionic monomers; ionic dimers; non-ionic monomers and non-ionic dimers. Older agents were generally ionic monomers with a relatively high osmolality and chemotoxicity. Non-ionic agents were developed in an attempt to overcome the adverse events associated with such media. Omnipaque is a non-ionic, monomeric agent which has a relatively low osmolality and chemotoxicity.
The blood-brain barrier separates the intravascular fluid space and the extracellular fluid space within the brain. This anatomic separation is thought to be the result of specialized tight junctions between the endothelial cells of so-called “continuous” capillaries and the absence of trans-endothelial vesicular transport [14].
Alteration of the blood-brain barrier may be related to the route of administration and the volume and speed at which the contrast agent is injected, its chemical structure and/or osmolality [15, 16]. Less blood-brain barrier alteration is observed when the contrast media are well diluted prior to reaching the cerebral vasculature as seen during intravenous administration as opposed to intra-arterial or intrathecal administration or with coronary angiography when compared to cerebral angiography. Those who have described the occurrence of transient cortical blindness after coronary angiography have reported using from 75 to 400 mL of contrast agent [17]. Only 75 mL was used in the case described here. Although non-ionic agents are thought to be less toxic than their ionic counterparts, and were indeed developed with this in mind, several cases of transient cortical blindness have now been reported with use of these relatively low-osmolality agents. Indeed, morbidity from neurological sequelae appears to be the same with ionic and non-ionic contrast media [12].
Duration of contact with the endothelium has been shown to be closely related to disruption of the blood-brain barrier [18]. Following the injection of solutions of higher viscosity than blood such as contrast media, there is a transient decrease in downstream blood flow [19]. The resultant increase in contrast transit time lengthens the duration of exposure of the cerebrovascular endothelium to the injected contrast media. Further, the patient’s prolonged supine posture during coronary angiography and the greater density of the contrast media relative to blood see the delayed clearance of contrast from the occipital lobes [20].
Contrast-induced cortical blindness likely overlaps with posterior reversible leukoencephalopathy syndrome. The latter is another uncommon entity with associations to include hypertension, renal insufficiency, neoplasms, post-transplant immunosuppression and, as here, the use of contrast [21]. The first of these is most commonly associated.
It is thought that the auto-regulatory capacity of the cerebral vessels is exceeded, following a sudden increase in systemic blood pressure for example. Breakdown of the blood-brain barrier with the focal transudation of fluid follows. In approximately 90% of cases, as in the case described here, the neurological deficits produced are reversible. The edematous lesions created may resolve upon control of blood pressure or reduction of immunosuppressive drugs.
The occipital cortex may be most vulnerable because of the relative lack of sympathetic innervation of the arterial system in this region and so also the relatively reduced “protective” sympathetic mediated arteriolar vasoconstriction [22]. Indeed, this is already thought to account for the predominance of posterior hemisphere lesions in eclampsia and hypertensive encephalopathy [23].
While in most of the reports of transient cortical blindness thought secondary to contrast-related neurotoxicity, many of the patients were known to have had hypertension, the details of patient blood pressure changes during angiography were often not elaborated.
The endothelins (ETs), ET-1, ET-2 and ET-3 are released by endothelial cells as well as non-vascular tissues, including the brain, kidney and lung. They have been shown to increase the permeability of human brain endothelial cells [24] and so are implicated in the pathophysiology of disorders like posterior reversible leukoencephalopathy syndrome. The administration of large volumes of radio-contrast medium to both animals and humans is associated with elevated ET levels [13]. Further, pre-existing endothelial dysfunction might be expected in a significant proportion of patients with cardiovascular disease or at least risk factors for the same undergoing coronary angiography.
The commonest causes of cortical blindness following coronary angiography are given in Table 1.
 Click to view | Table 1. Commonest Causes of Cortical Blindness Following Coronary Angiography |
Investigations
Should cortical blindness occur following coronary angiography, prompt neurological and ophthalmological reviews are warranted. Neuroimaging as described here is also indicated.
Treatment
Unfortunately, no specific measures may be undertaken in an attempt to prevent the unusual but alarming complication of cortical blindness following angiography. Heparin prophylaxis may indeed be useful but, as our case illustrates, does not totally avoid the risk of thrombosis and/or embolism associated with the use of guide wires and contrast media [12].
The patient’s blood pressure should be appropriately controlled. Our patient was anti-coagulated. This seemed a reasonable course of action given that occipital lobe infarction was thought to be the most likely diagnosis. A benefit for anticoagulation in cases of contrast-induced cortical blindness has not been proven. Further, there is no evidence to suggest that additional treatments, e.g. corticosteroids improve the natural history of this condition. Forced diuresis with the hope of removing the offending contrast agent has also not been shown to be beneficial.
Outcome and follow-up
The prognosis of patients who suffer transient cortical blindness during or following angiography is, not surprisingly, dependent upon the mechanism by which the neurological deficit develops. Reports of blindness thought to be due to contrast neurotoxicity suggest a generally favorable outcome. In most cases, the return of vision is gradual, proceeding from light and motion perception to an eventual return of colour vision. When the contrast medium has been excreted, which takes, on average, 3 days (range from 15 min to 3 weeks), “normal” vision returns as the protective function of the blood-brain barrier is restored [17].
The prognosis from other causes, such as emboli, may be less favorable [25] though admittedly not in this case.
Importantly, contrast-related breakdown of the blood-brain barrier appears to be sporadic. Re-exposure to contrast medium does not seem to reproduce the clinical effects of the previous exposure. There is however limited reported experience of re-challenging with contrast patients with a history of the same. In one report, re-exposure to contrast media during coronary angiography in three patients who had developed transient cortical blindness at a previous angiographic procedure did not lead to cortical blindness [26]. Nevertheless, it is worth considering pre-treatment with corticosteroids and attempting to minimize the amount of dye used during re-exposure [27].
Conclusion
Cortical blindness is a rare but alarming complication of coronary angiography. It has not yet been systematically studied in a prospective fashion and so reliable data on its frequency are absent. In the case described here, isolated occipital lobe emboli produced a clinical picture identical to that of a transient neurotoxic effect of the contrast media used. Initially, there was radiological evidence for neither. The outcome may be more favorable when caused by contrast-related neurotoxicity than when the condition follows true occipital lobe infarction.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
EMcE: data collection and interpretation; drafting of manuscript. DG: figure construction; drafting of manuscript; manuscript submission. KA: figure construction; drafting of manuscript. TK: co-ordination of manuscript construction; critical revision of manuscript. All authors read and approved the final manuscript.
Abbreviations
CT: computed tomography; ET-1: endothelin-1; ET-2: endothelin-2; ET-3: endothelin-3; MRI: magnetic resonance imaging
| References | ▴Top |
- de Bono D. Complications of diagnostic cardiac catheterisation: results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. The Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London. Br Heart J. 1993;70(3):297-300.
doi pubmed - Henzlova MJ, Coghlan HC, Dean LS, Taylor JL. Cortical blindness after left internal mammary artery to left anterior descending coronary artery graft angiography. Cathet Cardiovasc Diagn. 1988;15(1):37-39.
doi pubmed - Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness. An unusual complication of coronary angiography. Neurology. 1970;20(4):353-355.
doi pubmed - Kinn RM, Breisblatt WM. Cortical blindness after coronary angiography: a rare but reversible complication. Cathet Cardiovasc Diagn. 1991;22(3):177-179.
doi - De Wispelaere JF, Trigaux JP, Van Beers B, Gilliard C. Cortical and CSF hyperdensity after iodinated contrast medium overdose: CT findings. J Comput Assist Tomogr. 1992;16(6):998-999.
doi pubmed - Eckel TS, Breiter SN, Monsein LH. Subarachnoid contrast enhancement after spinal angiography mimicking diffuse subarachnoid hemorrhage. AJR Am J Roentgenol. 1998;170(2):503-505.
doi pubmed - Ballerini L, Barbaresi F, Binaghi G, Cernigliaro C, Chioin R, Fattori R, Inglese L, et al. Iopamidol in cardioangiography: a retrospective, multicenter study. Part I. Adult patients. Int J Card Imaging. 1992;8(1):35-43.
doi pubmed - Borghi C, Saia F, Marzocchi A, Branzi A. The conundrum of transient cortical blindness following coronary angiography. J Cardiovasc Med (Hagerstown). 2008;9(10):1063-1065.
doi pubmed - Hamon M, Gomes S, Clergeau MR, Fradin S, Morello R. Risk of acute brain injury related to cerebral microembolism during cardiac catheterization performed by right upper limb arterial access. Stroke. 2007;38(7):2176-2179.
doi pubmed - Skinner JS, Jackson MJ, Gholkar A, Adams PC. Cortical blindness during left internal mammary angiography. Int J Cardiol. 1995;52(2):119-123.
doi - Kido DK, King PD, Manzione JV, Simon JH. The role of catheters and guidewires in the production of angiographic thromboembolic complications. Invest Radiol. 1988;23(Suppl 2):S359-365.
doi pubmed - Davidson CJ, Mark DB, Pieper KS, Kisslo KB, Hlatky MA, Gabriel DA, Bashore TM. Thrombotic and cardiovascular complications related to nonionic contrast media during cardiac catheterization: analysis of 8,517 patients. Am J Cardiol. 1990;65(22):1481-1484.
doi - Zwicker JC, Sila CA. MRI findings in a case of transient cortical blindness after cardiac catheterization. Catheter Cardiovasc Interv. 2002;57(1):47-49.
doi pubmed - Sage MR, Wilson AJ. The blood-brain barrier: an important concept in neuroimaging. AJNR Am J Neuroradiol. 1994;15(4):601-622.
pubmed - Fischer HW. Occurrence of seizure during cranial computed tomography. Radiology. 1980;137(2):563-564.
doi pubmed - Scott WR. Seizures: a reaction to contrast media for computed tomography of the brain. Radiology. 1980;137(2):359-361.
doi pubmed - Yazici M, Ozhan H, Kinay O, Kilicaslan B, Karaca M, Cece H, Biceroglu S, et al. Transient cortical blindness after cardiac catheterization with iobitridol. Tex Heart Inst J. 2007;34(3):373-375.
pubmed - Rapoport SI, Fredericks WR, Ohno K, Pettigrew KD. Quantitative aspects of reversible osmotic opening of the blood-brain barrier. Am J Physiol. 1980;238(5):R421-431.
pubmed - Morris TW, Kern MA, Katzberg RW. The effects of media viscosity on hemodynamics in selective arteriography. Invest Radiol. 1982;17(1):70-76.
doi pubmed - Parry R, Rees JR, Wilde P. Transient cortical blindness after coronary angiography. Br Heart J. 1993;70(6):563-564.
doi pubmed - Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
doi pubmed - Merchut MP, Richie B. Transient visuospatial disorder from angiographic contrast. Arch Neurol. 2002;59(5):851-854.
doi pubmed - Heistad DD. Protection of the blood-brain barrier during acute and chronic hypertension. Fed Proc. 1984;43(2):205-209.
pubmed - Stanimirovic DB, Bertrand N, McCarron R, Uematsu S, Spatz M. Arachidonic acid release and permeability changes induced by endothelins in human cerebromicrovascular endothelium. Acta Neurochir Suppl (Wien). 1994;60:71-75.
doi - Thomas TD, Troost BT. Permanent cerebral blindness after cardiac catheterization. Cathet Cardiovasc Diagn. 1989;17(4):228-230.
doi - Rama BN, Pagano TV, DelCore M, Knobel KR, Lee J. Cortical blindness after cardiac catheterization: effect of rechallenge with dye. Cathet Cardiovasc Diagn. 1993;28(2):149-151.
doi pubmed - Frantz-W M. Cortial blindness following coonary angiography in a patient with LIMA bypass graft and end stage renal failure. Proceedings of EuroPCR: Paris. 2006;21-24.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.








