| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 5, Number 4, April 2014, pages 248-252
Terlipressine Induced Rhabdomyolysis After Orthotopic Liver Transplantation
Luminita Eida, b, Yoram G Weissa
aDepartment of Anesthesiology and Critical Care Medicine, Hadassah-Hebrew University Medical Center, Kiryat Hadassah, PO Box 12000, Jerusalem 91120, Israel
bCorresponding author: Luminita Eid, Department of Anesthesiology and Critical Care Medicine, Hadassah-Hebrew University Medical Center, Kiryat Hadassah, PO Box 12000, Jerusalem 91120, Israel
Manuscript accepted for publication February 4, 2014
Short title: Rhabdomyolisis induced by Terlipressine Administration
doi: https://doi.org/10.14740/jmc1721w
| Abstract | ▴Top |
In this report we present a fatal case of severe rhabdomyolysis during early recovery following a complicated but successful orthotopic liver transplantation. The key point in this case is skin necrosis and we consider that the clinical association of rhabdomyolysis and skin necrosis is a consequence of terlipressine induced severe vasoconstriction. A literature search was performed, in an attempt to identify similar cases as well as other plausible explanations.
Keywords: Liver cirrhosis; Orthotopic liver transplantation; Rhabdomyolisis; Myopathy; Vasopressin; Skin necrosis
| Introduction | ▴Top |
Rhabdomyolysis, the release of muscle cell content into the plasma has a complex etiology [1, 2]. Myoglobin, a low-molecular-mass protein found in striated muscles, is released within a few hours after injury [1]. Diagnosis is made by plasma increase of the specific muscle enzyme (creatine phosphakinase, CPK-MM), as well as myoglobinemia and myoglobinuria [2]. Urinary and serum levels of myoglobin are unrelated to each other or to the development of renal failure [3].
In this case report we present a fatal case of rhabdomyolysis in a newly transplanted patient. We performed a literature search, in an attempt to identify the possible causes of rhabdomyolysis and discuss the pathophysiological mechanisms. Three major causes were identified as possible triggers: terlipressine, propofol and cirrhotic myopathy. As for the other suspected factors: electrolyte dissembalance, prolonged immobilization, some inherited mitochondrial defect in lipid metabolism, a newly and not fully recovered liver, we think they were minor factors that may have contributed to the negative outcome but were not solely responsible for it.
| Case Report | ▴Top |
A 51 years old patient with HCV liver cirrhosis and an MELD score of 30 underwent liver transplantation. Three months previously, during a gastrointestinal bleeding episode she was treated with terlipressine. Complaints of chest pain, hypertension, bradycardia and fingertips paresthesias were reported both after an i.v. bolus and the continuous administration. The treatment was stopped.
The patient received a good liver. During the dissection stage, due to massive bleeding and consequent hemodynamic instability, the patient required large quantities of blood and inotropic support. Reperfusion of the liver underwent smoothly. The patient was transferred to the ICU. Propofol 1 - 2 mg/kg/h and remifentanyl 1 - 2 µg/kg/h were started for sedation. Mechanical ventilation, and inotropic support were continued. Duplex ultrasound of the liver showed normal portal and hepatic artery blood flows. During the following hours liver enzymes, metabolic acidosis and coagulation profile improved suggesting a functioning liver.
During the following days, the patient’s condition improved. On postoperative day (POD) 3, she was fully awake, hemodynamically stable with adequate urinary output, minimal ventilatory support and good liver function. New onset gastrointestinal bleeding required transfusion of 4 units of blood. Gastroscopy showed multiple oozing points in the gastric mucosa. Two doses of terlipressine acetate, 1 mg each, were administered at 4 h interval. Several hours later, severe skin ischemia was noted on arms, legs, thighs and abdomen followed by onset of purple bullous lesions together with massive edema (Fig. 1, 2). Urinalysis revealed myoglobinuria while serum CPK increased to 31,000 U/L (Table 1). A diagnosis of severe rhabdomyolysis together with skin necrosis was made. Soon thereafter, the patient became unstable requiring escalating doses of inotropic support. Severe metabolic acidosis with hyperkalemia and anuric renal failure required dialysis. Acute lung injury and deterioration in liver function were noted. Despite aggressive treatment the patient’s condition continued to deteriorate and she succumbed on POD 9 due to multiple organ failure and superimposed sepsis.
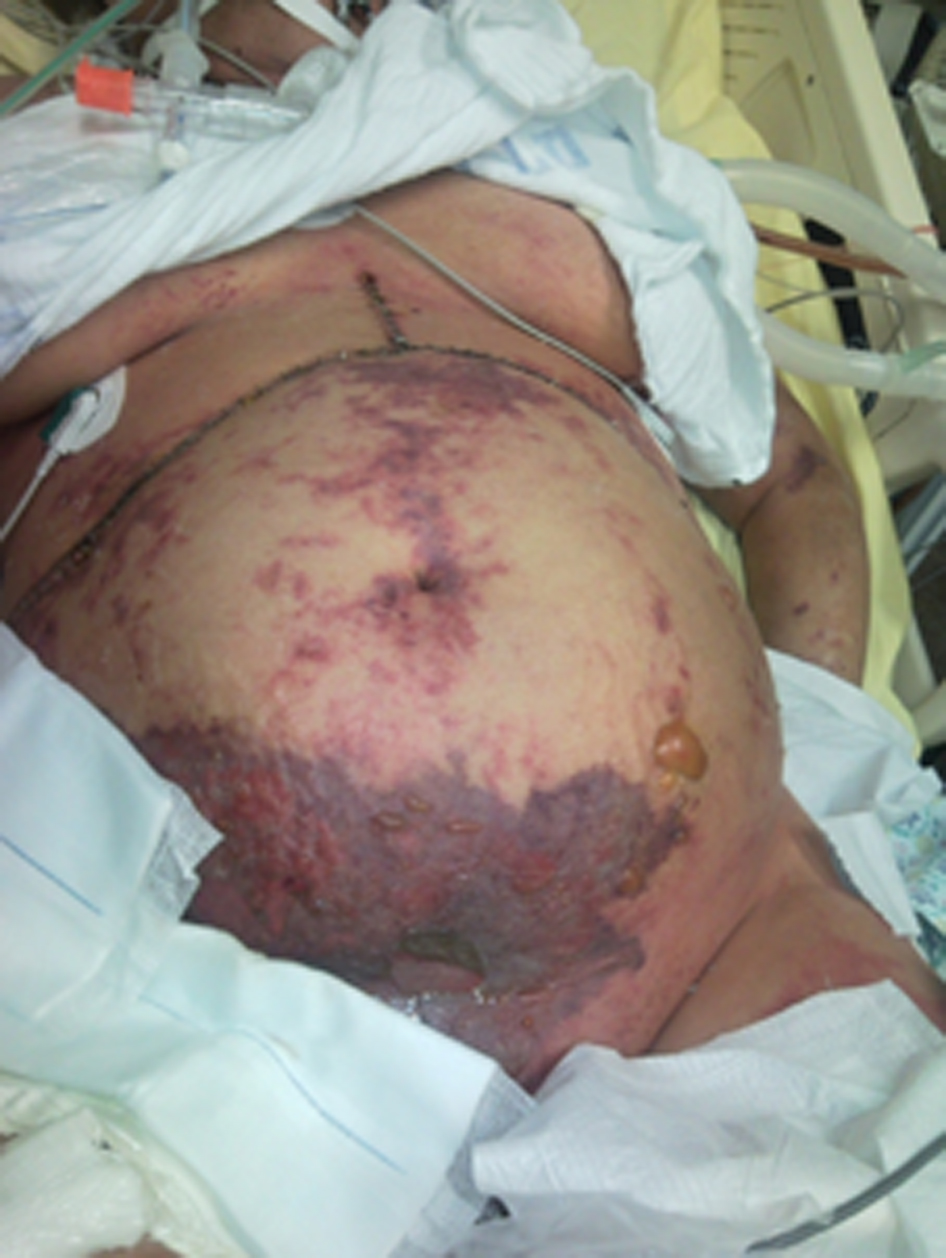 Click for large image | Figure 1. Extended area of severe skin ischemia and massive edema may be noticed on the arms, legs, thighs and abdomen together with purple bullous lesions. |
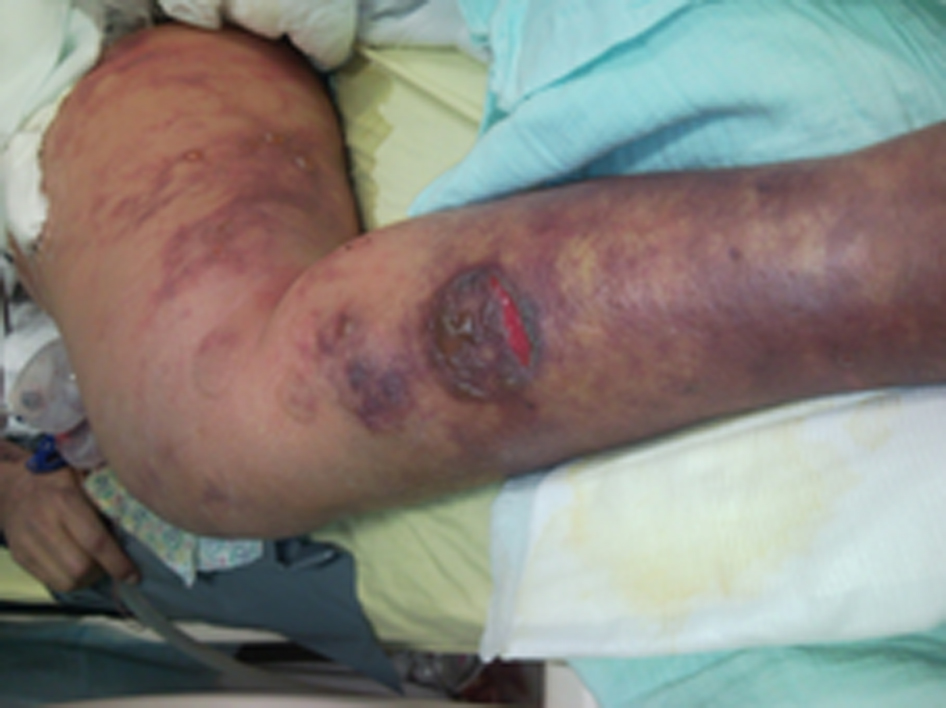 Click for large image | Figure 2. These lesions are considered secondary to intense peripheral vasoconstriction induced by terlipressine administration. |
| Discussion | ▴Top |
After reviewing this case three potential causes of rhabdomyolysis were identified and they are discussed below.
Vasopressine induced rhabdomyolysis
Terlipressin (Glypressin® - Ferring Pharmaceuticals - triglycyl-lysine vasopressin) is a vasopressin analog [4] used as a first-line drug for esophageal bleeding in cirrhotic patients with type 1 hepatorenal syndrome (HRS) [5, 6] because of its beneficial effect on renal perfusion and glomerular filtration rate (reduces hepatic venous pressure gradient, variceal pressure and azygos blood flow) [7].
Cleavage of the N-triglycyl residue in the terlipressin molecule by endothelial peptidases results in a “slow release” of the vasoactive lysine vasopressin [4]. The half-life of terlipressin is 6 h whereas that of vasopressin is only 6 min. The therapeutic effect is due to its binding to the V1 and V2 receptors on the vascular smooth muscle cells of the portal vessels followed by intense vasoconstriction, thus hindering bleeding from these vessels [4]. Because of V2 receptors activation on endothelial cells, causing von Willebrand factor release, it enhances platelet aggregation and therefore the risk of thrombosis [4].
Terlipressin proved to be safe, with a low incidence of side-effects (usually mild: headache, abdominal pain, diarrhea, bradycardia, increase in blood pressure) [8]. More serious complications, primarily cardiac ischemia, are uncommon, but have been reported [9]. Ischemic effects are described in the literature, affecting intestinal mucosa, skin, distal limbs, and genitalia [10]. Atypical sites of necrosis are also cited: the scalp, tongue, foreskin, scrotum and breasts [11]. Rhabdomyolysis is frequently associated with ischemia and worsens outcome [12].
Nevertheless, when the drug was administered for the first time in our patient, the side-effects proved to be difficult to tolerate and the treatment was aborted. After the second administration, skin ischemia was noted followed by appearance of purple bullous lesions and massive edema in the same area (similar to the sites described in the literature) [12, 13]. Soon after, myoglobinuria and high CPK were detected together with severe metabolic acidosis, hyperkalemia and anuric renal failure.
There is no known association between the severity of skin ischemia and the dose or the length of terlipressin administration [13]. Sepsis related hypoperfusion, concomitant administration of catecholamines or steroids [14] and arterial catheterization [15] may act as additional risk factors. Although our patient was not obese, edema was present in the lower part of the body, stretching the skin of the abdomen and lower limbs and increasing the surface area for the microvascular blood supply. The use of terlipressin in the presence of low tissue oxygen levels may have led to ischemic complications [16]. Moreover, relative hypovolemia associated with early recovery after liver transplantation together with concomitant administration of steroids (as part of immunosuppression) may have enhanced terlipressin effect [14].
Propofol infusion syndrome (PIS)
PIS is a devastating clinical entity associated with propofol administration. Propofol (Diprivan® - Astra Zeneca) a central-acting diisopropyl phenol compound, gained acceptance due to its ultrashort onset and duration of action [17]. Although initially recognized in children, PIS is now accepted to occur in adults also. It has been described as an “all or none” syndrome with sudden onset and likely death [18].
A large spectrum of findings have been associated with PIS: myocardial failure, brady-arrhythmias, ST-segment changes, lipemia, fatty liver, rhabdomyolysis, lactic acidosis, hyperkalemia, acute renal failure, and metabolic acidosis [19]. This may be the result of cumulative toxicity, with reports coming after high-dose infusions as well as after prolonged administration [20]. Risk factors for the PIS include: large cumulative doses, young age, acute neurological injury, low carbohydrate intake, high fat intake or inadequate clearance, exogenous catecholamine or corticosteroid infusion, critical illness and inborn errors of mitochondrial fatty acid oxidation [21].
Current guidelines for maximal propofol dosage suggested < 4 mg/kg//h for a duration of up to 48 h [22]. Recently, fatal cases of PIS at low infusion rates (1.9 - 5.1 mg/kg/h) have been reported [23]. Higher propofol doses during shorter periods of time can also trigger the syndrome [24]. In our case propofol sedation was started after surgery and continued for the first three postoperative days but the doses did not exceed 2 mg/kg/h, being even below the minimal problematic dose [25, 26].
The mechanism underlying PIS has yet to be clarified. Some evidence points to an abnormality of fatty acid metabolism suggested by elevated carnitine levels [27]. Pathology studies on patients that died of PIS found diffuse myonecrosis in both cardiac and skeletal muscle [28]. Muscle cells may be susceptible to propofol because of their high ATP requirements and reliance on the fatty acids oxidation to meet their energy needs [29]. PIS is similar to the inherited mitochondrial myopathies, often clinically silent until a metabolic stress is encountered, when the body comes to rely on fat rather than carbohydrate for its energy requirements [27].
Although low glycogen stores may have existed in this severely wasted patient, a normal carbohydrate intake of 3.5 g/kg/day was assured [21]. Nothing is known about inborn errors of mitochondrial fatty acid oxidation in the recipient or in the transplanted liver. The blockade of the fatty acid metabolism by propofol together with the fat load delivered by the propofol emulsion in the presence of a newly transplanted and not yet fully recovered liver may have overwhelmed cellular functions and induced cytotoxic effects. However, if rhabdomyolysis may be explained by the PIS, extensive skin necrosis, a specific feature in this case is not a component of the syndrome.
Idiopathic rhabdomyolysis
Although myopathy associated with liver cirrhosis has not been established as a disease entity [30], idiopathic cases of rhabdomyolysis have been reported. The mortality rate in this group is high, 31% vs. 10-12% in the general population [31]. Another feature is the recurrence of these episodes as well as the lack of any obvious causes [32]. Many cirrhotic patients have muscular symptoms (cramps, weakness and tenderness which may or may not be associated with rhabdomyolysis) [33], but little is known about the mechanism of idiopathic recurrent rhabdomyolysis [34]. It may be related to altered metabolism due to hepatic dysfunction [34].
Even though not a disease “per se”, acute myopathy can develop in liver cirrhosis [31]. A subclinical myopathy cannot be excluded in our patient (muscle biopsies were not performed), but no specific muscular symptoms or laboratory signs suggestive for rhabdomyolysis were recorded at any time before transplantation.
Conclusion
In this report we presented a case of lethal rhabdomyolysis after liver transplantation. After analyzing the clinical picture and based on the temporal sequence, we believe that the culprit was terlipressin injection. The key point of this case is skin necrosis. It is not associated with rhabdomyolysis in any of the above mentioned situations except vasopressin or analogs administration. Moreover, the chain of events that led to terlipressine administration and the fatal outcome is also significant for this case.
It is possible that some other risk factors may have contributed to rhabdomyolysis and led to the final outcome: hypovolemia with moderate electrolyte disorder, a subclinical cirrhotic myopathy, infection, immunosuppressive treatment and propofol even in small doses. Medical miscommunication, the failure to convey relevant medical information between key players in the medical team held an important part in this case [35]. In hospital systems, medical notes are supposed to be transferred internally from department to department [36]. The information relevant for the initial reaction to terlipressine administration (chest pain, hypertension, bradycardia, fingertips paresthesias and pulmonary congestion) was not passed as important to the intensive care team but as merely a note in an overloaded hospitalization chart, and thus, overlooked. We believe it should have been categorized as severe drug allergy/reaction and listed accordingly. Thus, it would have been noticed and a major negative event prevented. In this new light, terlipressin administration was impetuous and it came at a high cost.
This case also led to a change in our approach towards cirrhotic patients - cirrhotic myopathy is looked for during initial evaluation, electrolyte abnormalities are corrected more aggressively and team rather than individual decisions are employed whenever new medication should be added or old one changed. Medical records of the transplant patients, covering sometimes years of follow-up, treatments and their response, are periodically reviewed and updated.
We consider this event to be a lethal complication of terlipressin administration. Although the literature is sparse, the clinical picture fitted previous descriptions of such atypical cases. From this experience one can learn that potent drugs such as terlipressin should only be administered for their absolute indications. Even when adverse reactions are rare, when they do occur they may be catastrophic.
Conflict of Interest
This case was a routine transplantation case. No grant was used for material preparation and submission. No conflict of interest exists between the authors or the authors and the pharmaceutical companies or drug suppliers.
| References | ▴Top |
- Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11(8):1553-1561.
pubmed - Allison RC, Bedsole DL. The other medical causes of rhabdomyolysis. Am J Med Sci. 2003;326(2):79-88.
doi - Delpassand ES, Dhekne RD, Barron BJ, Moore WH. Evaluation of soft tissue injury by Tc-99m bone agent scintigraphy. Clin Nucl Med. 1991;16(5):309-314.
doi pubmed - Kam PC, Williams S, Yoong FF. Vasopressin and terlipressin: pharmacology and its clinical relevance. Anaesthesia. 2004;59(10):993-1001.
doi pubmed - Moreau R, Lebrec D. The use of vasoconstrictors in patients with cirrhosis: type 1 HRS and beyond. Hepatology. 2006;43(3):385-394.
doi pubmed - Halimi C, Bonnard P, Bernard B, Mathurin P, Mofredj A, di Martino V, Demontis R,
et al . Effect of terlipressin (Glypressin) on hepatorenal syndrome in cirrhotic patients: results of a multicentre pilot study. Eur J Gastroenterol Hepatol. 2002;14(2):153-158.
doi pubmed - Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet. 1995;346(8979):865-868.
doi - Megarbane H, Barete S, Khosrotehrani K, Izzedine H, Moguelet P, Chosidow O, Frances C,
et al . Two observations raising questions about risk factors of cutaneous necrosis induced by terlipressin (Glypressin). Dermatology. 2009;218(4):334-337.
doi pubmed - Rosario R, Lalanne B, Lebre P, Lepesan D, Martelet JP, Dupont M, Camatte R,
et al . [Myocardial infarction after injection of terlipressin for digestive hemorrhage]. Gastroenterol Clin Biol. 1996;20(8-9):712-713.
pubmed - Borrego R, Lopez-Herce J, Mencia S, Carrillo A, Sancho L, Bustinza A. Severe ischemia of the lower limb and of the intestine associated with systemic vasoconstrictor therapy and femoral arterial catheterization. Pediatr Crit Care Med. 2006;7(3):267-269.
doi pubmed - Donnellan F, Cullen G, Hegarty JE, McCormick PA. Ischaemic complications of Glypressin in liver disease: a case series. Br J Clin Pharmacol. 2007;64(4):550-552.
doi pubmed - Moreno-Sanchez D, Casis B, Martin A, Ortiz P, Castellano G, Munoz MT, Vanaclocha F,
et al . Rhabdomyolysis and cutaneous necrosis following intravenous vasopressin infusion. Gastroenterology. 1991;101(2):529-532.
pubmed - Di Micoli A, Bracci E, Cappa FM, Casadio R, Zambruni A, Fontana K, Bernardi M,
et al . Terlipressin infusion induces ischemia of breast skin in a cirrothic patient with hepatorenal syndrome. Dig Liver Dis. 2008;40(4):304-305.
doi pubmed - Dunser MW, Mayr AJ, Tur A, Pajk W, Barbara F, Knotzer H, Ulmer H,
et al . Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med. 2003;31(5):1394-1398.
doi pubmed - Vaccaro F, Giorgi A, Riggio O, De Santis A, Laviano A, Rossi-Fanelli F. Is spontaneous bacterial peritonitis an inducer of vasopressin analogue side-effects? A case report. Dig Liver Dis. 2003;35(7):503-506.
doi - Obritsch MD, Bestul DJ, Jung R, Fish DN, MacLaren R. The role of vasopressin in vasodilatory septic shock. Pharmacotherapy. 2004;24(8):1050-1063.
doi pubmed - Rosen DJ, Nicoara A, Koshy N, Wedderburn RV. Too much of a good thing? Tracing the history of the propofol infusion syndrome. J Trauma. 2007;63(2):443-447.
doi pubmed - Fodale V, La Monaca E. Propofol infusion syndrome: an overview of a perplexing disease. Drug Saf. 2008;31(4):293-303.
doi - Fudickar A, Bein B, Tonner PH. Propofol infusion syndrome in anaesthesia and intensive care medicine. Curr Opin Anaesthesiol. 2006;19(4):404-410.
doi pubmed - Short TG, Young Y. Toxicity of intravenous anaesthetics. Best Pract Res Clin Anaesthesiol. 2003;17(1):77-89.
doi - Orsini J, Nadkarni A, Chen J, Cohen N. Propofol infusion syndrome: case report and literature review. Am J Health Syst Pharm. 2009;66(10):908-915.
doi pubmed - Barr J, Egan TD, Sandoval NF, Zomorodi K, Cohane C, Gambus PL, Shafer SL. Propofol dosing regimens for ICU sedation based upon an integrated pharmacokinetic-pharmacodynamic model. Anesthesiology. 2001;95(2):324-333.
doi pubmed - Marinella MA. Lactic acidosis associated with propofol. Chest. 1996;109(1):292.
doi pubmed - Liolios A, Guerit JM, Scholtes JL, Raftopoulos C, Hantson P. Propofol infusion syndrome associated with short-term large-dose infusion during surgical anesthesia in an adult. Anesth Analg. 2005;100(6):1804-1806.
doi pubmed - FDA issues warning on propofol (Diprivan). Can Med Assoc J. 2001;164:160838.
- Merz TM, Regli B, Rothen HU, Felleiter P. Propofol infusion syndrome—a fatal case at a low infusion rate. Anesth Analg. 2006;103(4):1050.
doi pubmed - Wolf A, Weir P, Segar P, Stone J, Shield J. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet. 2001;357(9256):606-607.
doi - Stelow EB, Johari VP, Smith SA, Crosson JT, Apple FS. Propofol-associated rhabdomyolysis with cardiac involvement in adults: chemical and anatomic findings. Clin Chem. 2000;46(4):577-581.
pubmed - Schenkman KA, Yan S. Propofol impairment of mitochondrial respiration in isolated perfused guinea pig hearts determined by reflectance spectroscopy. Crit Care Med. 2000;28(1):172-177.
doi - Abrams GA, Concato J, Fallon MB. Muscle cramps in patients with cirrhosis. Am J Gastroenterol. 1996;91(7):1363-1366.
pubmed - Baek JE, Park DJ, Kim HJ, Lee JD, Chang SH. The clinical characteristics of rhabdomyolysis in patients with liver cirrhosis. J Clin Gastroenterol. 2007;41(3):317-321.
doi pubmed - Black C, Jick H. Etiology and frequency of rhabdomyolysis. Pharmacotherapy. 2002;22(12):1524-1526.
doi - Konikoff F, Theodor E. Painful muscle cramps. A symptom of liver cirrhosis?. J Clin Gastroenterol. 1986;8(6):669-672.
doi - Lee OJ, Yoon JH, Lee EJ, Kim HJ, Kim TH. Acute myopathy associated with liver cirrhosis. World J Gastroenterol. 2006;12(14):2254-2258.
pubmed - Murphy JG, Dunn WF. Medical errors and poor communication. Chest. 2010;138(6):1292-1293.
doi pubmed - Reader TW, Flin R, Cuthbertson BH. Communication skills and error in the intensive care unit. Curr Opin Crit Care. 2007;13(6):732-736.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.








